Water soluble puerarin derivatives and preparation method and application thereof
A technology of puerarin derivatives and puerarin, which is applied in the direction of sugar derivatives, chemical instruments and methods, compounds of group 5/15 elements of the periodic table, etc., and can solve problems such as unstable preparations and poor water solubility of puerarin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
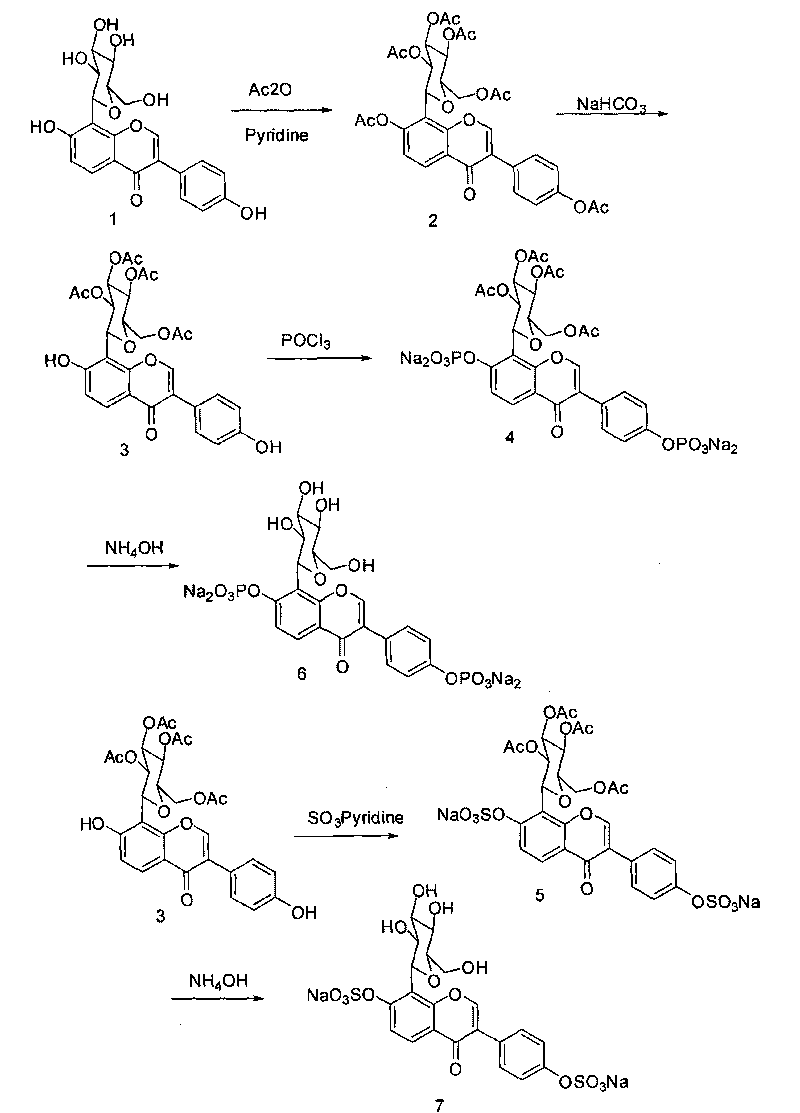
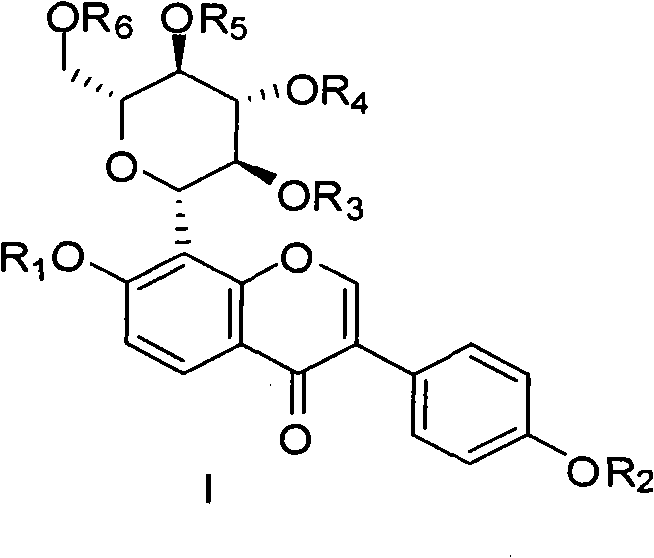
[0055] Dissolve 4.5 g (10.8 mmol) of puerarin (expressed in Formula 1) in 30 mL of dry pyridine, and slowly add 7.5 mL of acetic anhydride with stirring at room temperature. Stirring was continued at room temperature for 24 hours. Pyridine was evaporated under reduced pressure, and dilute HCl was added to adjust the pH to about 6. Use ethyl acetate, continue to stir for 24 hours, TLC tracking until the raw material point disappears (developing agent dichloromethane:methanol 9:1), evaporate pyridine under reduced pressure, add dilute HCl aqueous solution to adjust the pH value to 6, and extract with ethyl acetate , MgSO 4 After drying, filter, evaporate to dryness ethyl acetate to obtain crude product, column chromatography, eluent is dichloromethane:methanol=25:1, collect and evaporate to dryness to obtain 5.05g white powder acetyl puerarin (shown in formula 2). H1NMR (CDCl3): δ8.20(d, J=8.0Hz, 1H), 8.00(s, 1H), 7.57(d, J=8.4Hz, 2H), 7.17(d, J=8.4Hz, 2H), 7.0(d, J=8.4HZ, 1H...
Embodiment 2
[0057] At room temperature, dissolve 5 g (7.48 mmol) of white powder acetyl puerarin (expressed in formula 2) in 100 mL of dry dichloromethane, slowly add 30 mL of saturated sodium bicarbonate solution and stir, TLC traces to the raw material Point disappears, adding appropriate amount of water, dichloromethane extraction, anhydrous Na 2 SO 4 After drying, it was filtered, and the filtrate was evaporated to dryness to obtain a crude product, which was purified by column chromatography, and the elution condition was dichloromethane:methanol=45:1 to obtain 3.2 g of a white solid (shown in Formula 3), with a yield of 69.4%. H1NMR (CDCl3): δ8.30(br s, 1H), 8.21(d, J=8.8HZ, 1H), 7.99(s, 1H), 7.39(d, J=7.6HZ, 2H), 7.28(s, 1H), 7.03(d, J=8.8HZ, 1H), 6.89(d, J=7.6HZ, 2H), 5.99(br s, 1H), 5.48(m, 3H), 5.37(m, 1H), 4.40 (dd, J=8.0, 3.6HZ, 1H), 4.25(d, J=8.0Hz, 1H), 4.00(m, 1H), 2.16(s, 3H, 2, 12(s, 3H), 2.06(s , 3H), 1.71(s, 3H).
Embodiment 3
[0059] Dissolve 1 gram of tetraacetylpuerarin (compound shown in formula 3) in dry pyridine, stir at room temperature, add 1.57 grams of phosphorus oxychloride (POCl 3 ), stirred for 24 hours after the addition; traced by TLC until the disappearance of the raw material point (developing agent is dichloromethane:methanol=13:1, v / v), evaporated pyridine, added an appropriate amount of water, and used 2N NaOH solution as the eluent Neutralize to pH 7, separate the macroporous resin AB-8, elute with pure water and collect, and evaporate the water to dryness under reduced pressure to obtain 500 mg of tetrahydroxypuerarin phosphate white powder (shown in formula 4).
[0060] At room temperature, its solubility in water is greater than 200mg / 100ml. A certain amount of sample was mixed with rat anticoagulant plasma, incubated at 37°C, and the drug was extracted with acetonitrile at different time points for HPLC analysis. The half-life of puerarin sodium phosphate converted into puera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com