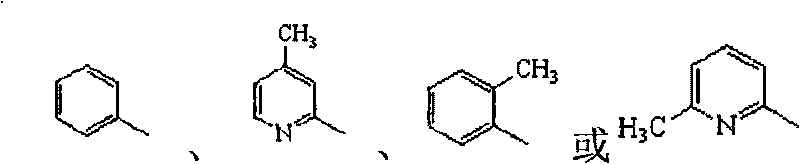
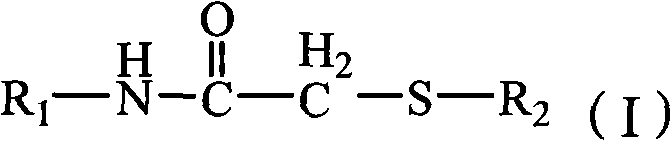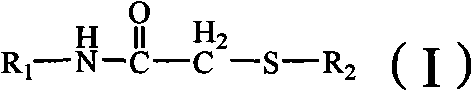2-amino-1,3,4-thiadiazolethioacetamido aromatic hydrocarbon derivatives, method for preparing same and use of same
A technology of thiadiazole thioacetamidoaromatics and thiadiazoles, which is applied in the field of 2-amino-1,3,4-thiadiazole thioacetamidoaromatics derivatives and their preparation and application, and can solve the high price of fungicides , high toxicity of fungicides, no reports and other issues, to achieve the effect of good dispersion and suspension, fast disintegration speed and accurate measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 2-(2-Amino-1,3,4-thiadiazole-2-thio)-N-o-methylphenylacetamide (L 1 )Synthesis
[0051]
[0052]Add 0.26g (2mmol) of 2-amino-5-mercapto-1,3,4-thiadiazole and 0.11g (2mmol) of KOH and 5mL of ethanol into a 25mL single-necked round-bottomed flask, heat slightly until completely dissolved, Add 0.40g (2.2mmol) of o-methyl chloride acetanilide, TLC tracking, react for 46h, slowly pour the reaction solution into 20mL of water, let stand for half an hour, filter with suction, recrystallize with 50% ethanol, and dry in vacuo to obtain White solid with a yield of 78.6% and a melting point of 178-179°C.
[0053] Characterization of product parameters:
[0054] IR(KBr) v: 3272, 1654, 1510, 1410, 1186, 738cm -1 ; 1H NMR (DMF): δ8.95 (1H, NHCO), 8.12 (1H, benzene ring C 6 -H), 7.28 (2H, benzene ring C 3 -H,C 5 -H,), 7.12 (1H, benzene ring C 4 -H), 6.70 (2H, NH 2 ), 4.12 (2H, CH 2 S), 2.41 (3H, CH 3 ); MS m / z 281.1 (M+H)+(calcd 280.1);.
Embodiment 2
[0056] 2-(2-Amino-1,3,4-thiadiazole-2-thio)-N-phenylacetamide (L 2 )Synthesis
[0057]
[0058] The synthesis method is the same as in Example 1, using 5-methyl-2-mercapto-1,3,4-thiadiazole to react with chloroacetanilide to obtain a white solid with a yield of 61.6%. Melting point: 174-175°C.
[0059] Characterization of product parameters:
[0060] IR(KBr) v: 3300, 1664, 1518, 1232, 674cm -1 ; 1H NMR (DMF): δ9.18 (1H, NHCO), 8.55 (1H, benzene ring C 6 -H), 7.99 (1H, benzene ring-H), 7.65 (1H,), 7.40 (1H, benzene ring-H), 7.15 (3H, NH 2 ), 4.12 (2H, CH 2 S), 2.41 (3H, CH 3 ).
Embodiment 3
[0062] 2-(2-Amino-1,3,4-thiadiazole-2-sulfur)-N-(4-methylpyridin-2-yl)acetamide (L 4 )Synthesis
[0063]
[0064] The synthesis method is the same as in Example 1, using 5-methyl-2-mercapto-1,3,4-thiadiazole and 4-methyl-2-chloroacetylaminopyridine to react to obtain a white solid with a yield of 64.3%, melting point : 248-249°C.
[0065] Characterization of product parameters:
[0066] IR(KBr)υ: 3372, 1666, 1570, 1188, 812cm -1 ; 1H NMR (DMF): δ9.95 (1H, s, NHCO), 8.65 (s, 1H, pyridine ring-H), 8.03-8.04 (m, 2H, pyridine ring-H), 7.24-7.28 (b, 2H, NH 2 ), 4.07 (s, 2H, CH 2 S), 2.68(s, 3H, CH 3 ); MSm / z 282.1(M+H)+(calcd 281.1)
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com