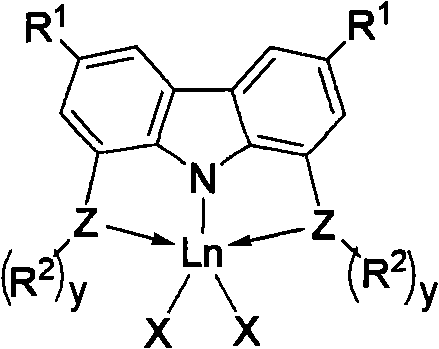
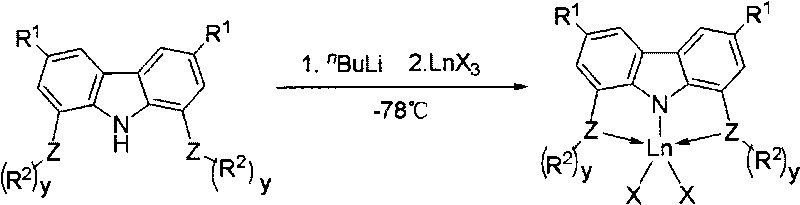Application of rare-earth complexes chelated by tridentate carbazolyl in conjugated diene and polar monomer copolymerization catalyst system
A technology of tridentate carbazolyl and rare earth complexes, applied to compounds of elements of group 4/14 of the periodic table, compounds of group 5/15 elements of the periodic table, compounds containing elements of group 3/13 of the periodic table, etc. direction, which can solve the problems of catalyst loss of activity, less research on conjugated dienes and polar monomers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0068] Complex Preparation Example 1 Preparation of Complex 1
[0069]
[0070] Under the protection of nitrogen, under the condition of -78°C, a hexane solution (0.34 mL, 0.50 mmol) of butyllithium with a concentration of 1.50 mol / L was added dropwise to 3,6-dimethyl-1,8-dibis In the 20mL tetrahydrofuran solution of tert-butyl sulfoyl carbazole (0.24g, 0.50mmol), continue to react for 1h after adding butyllithium, add ScCl 3 (0.075g, 0.5mmol) as a solid, after naturally warming to room temperature overnight. The tetrahydrofuran was removed by concentration, and the residue was extracted with toluene. After the toluene solution was concentrated, 0.15 g of complex 1 was obtained as a pale yellow solid, with a yield of 51.0%. Its molecular formula is C 32 h 46 Cl 2 NP 2 Sc, elemental analysis results (%): C, 60.05; H, 7.65; N, 2.24.
preparation Embodiment 2
[0071] Complex Preparation Example 2 Preparation of Complex 2
[0072]
[0073] At room temperature and under the protection of nitrogen, Dy(CH 2 SiMe 3 ) 3 (THF) 2 (0.28g, 0.50mmol) in 5ml tetrahydrofuran solution was added in 5 minutes in 3,6-dimethyl-1,8-bis-diphenylzanylcarbazole (0.28g, 0.50mmol) in 20mL tetrahydrofuran solution, After reacting at room temperature for 2 h, the residue was concentrated to 1 ml, and then 1-2 ml of n-hexane was added to obtain yellow solid complex 2, 0.26 g, with a yield of 57.8%. Its molecular formula is C 46 h 52 NP 2 Si 2 Dy, elemental analysis results (%): C, 61.33; H, 5.78; N, 1.49.
preparation Embodiment 3
[0074] Complex Preparation Example 3 Preparation of Complex 9
[0075]
[0076] At room temperature and under the protection of nitrogen, Y(CH 2 C 6 h 4 -o-N(CH 3 ) 2 ) 3 (0.25g, 0.50mmol) in 5ml tetrahydrofuran solution was added within 5 minutes to 3,6-di-tert-butyl-1,8-bisdiphenylsanylcarbazole (0.32g, 0.50mmol) in 20mL tetrahydrofuran solution , then reacted at 70°C for 2h and concentrated to 1ml, then added 1-2ml of n-hexane to obtain yellow solid complex 9, 0.34g, yield 68.0%. Its molecular formula is C 62 h 66 N 3 P 2 Y, elemental analysis results (%): C, 74.08; H, 6.57; N, 4.11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com