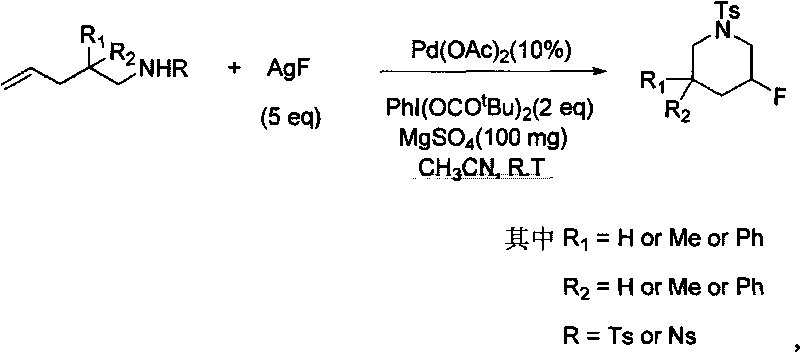
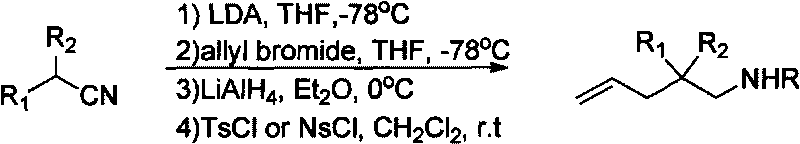Method for synthesizing fluoro nitrogen heterocyclic compound
A heterocyclic compound and fluorinated technology, applied in organic chemical methods, chemical instruments and methods, organic halogenation, etc., to achieve high yield, good selectivity, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0015] Example 1: Synthesis
[0016] 4.5mg (0.02mmol) of Pd(OAc) 2 , 162.5mg (0.4mmol) of PhI (OCO t Bu) 2 , 125.9mg (1.0mmol) of AgF, 100mg of MgSO 4 and olefins 53.4mg (0.2mmol) was added to the reaction tube, then 1ml of anhydrous acetonitrile was added, and stirred at room temperature for 24 hours. Then filter, the solid was washed with dichloromethane, the combined filtrate was concentrated, and column chromatography was used to obtain 47.9 mg of product with gradient elution of ethyl acetate and petroleum ether The yield was 84%.
[0017] 1 H NMR (400M, CDCl 3 , TMS), δ7.65(d, J=8.0Hz, 2H), 7.34(d, J=8.0Hz, 2H), 4.77(dm, J=47.6Hz, 1H), 3.61(ddd, J=13.6, 11.2, 3.6Hz, 1H), 2.62(ddd, J=11.2, 7.6, 7.6Hz, 1H), 2.97(d, J=11.2Hz, 1H), 2.44(s, 3H), 2.38(d, J=11.2 Hz, 1H), 1.72(ddd, J=16.8, 13.2, 4.0Hz, 1H), 1.35(ddd, J=12.8, 12.8, 8.8Hz, 1H), 1.03(d, J=3.6Hz, 6H). 13 C NMR (100M, CDCl 3 ), δ143.66, 133.24, 129.70, 127.45, 85.55(d, J=231.9Hz), 56.58(d, J=1.5Hz), 49...
example 2
[0018] Example 2: Synthesis of (without MgSO4)
[0019] 4.5mg (0.02mmol) of Pd(OAc) 2 , 162.5mg (0.4mmol) of PhI (OCO t Bu) 2 , 125.9 mg (1.0 mmol) of AgF, alkene 53.4mg (0.2mmol) was added to the reaction tube, then 1ml of anhydrous acetonitrile was added, and stirred at room temperature for 24 hours. Then filter, the solid is washed with dichloromethane, the combined filtrates are concentrated, column chromatography, and gradient elution with ethyl acetate and petroleum ether to obtain 39 mg of product The yield is 69%.
Embodiment 3
[0020] Example 3: Synthesis
[0021] 4.5mg (0.02mmol) of Pd(OAc) 2 , 162.5mg (0.4mmol) of PhI (OCO t Bu) 2 , 125.9mg (1.0mmol) of AgF, 100mg of MgSO 4 and olefins 59.7mg (0.2mmol) was added to the reaction tube, then 1ml of anhydrous acetonitrile was added, and stirred at room temperature for 24 hours. Then filter, the solid was washed with dichloromethane, the combined filtrate was concentrated, and column chromatography was used to obtain 46.8 mg of product with ethyl acetate and petroleum ether gradient washing method The yield was 74%.
[0022] 1 H NMR (400M, CDCl 3 , TMS), δ8.39(d, J=8.8Hz, 2H), 7.96(d, J=8.8Hz, 2H), 4.80(dm, J=47.2Hz, 1H), 3.56(ddd, J=16.0, 11.6, 4.0Hz, 1H), 2.97(d, J=11.6Hz, 1H), 2.89(ddd, J=11.6, 7.6, 7.6Hz, 1H), 2.61(d, J=12.0Hz, 1H), 1.71( ddd, J=20.4, 13.6, 4.0Hz, 1H), 1.46(ddd, J=12.8, 12.8, 8.0Hz, 1H), 1.03(s, 6H). 13 C NMR (100M, CDCl 3 ), δ150.18, 142.90, 128.57, 124.36, 85.38 (d, J = 175.5Hz), 56.57, 49.46 (d, J = 28.3Hz), 42.22 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com