Preparation method of dexrazoxane and pharmaceutical salts thereof
A kind of technology of delazox and medicinal salt, applied in the field of preparation of delazox or its pharmaceutically acceptable salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
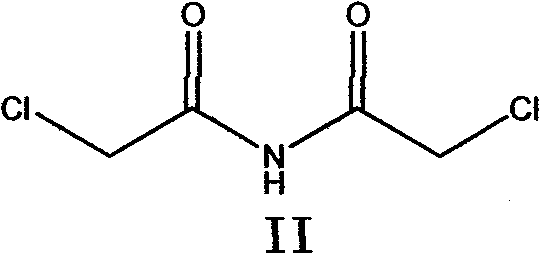
[0049] Add 1193g of chloroacetonitrile into a 3000ml three-necked reaction flask, add 3580g of saturated hydrogen chloride methanol solution, stir at 25°C for 8 hours, heat up to 125°C for 1 hour, cool to 30°C, pour into 3000ml of water, stir for 2 hours, filter, Purified with ethanol to obtain 843.7g of N,N-dichloroacetimide (II), mp=195-197°C.
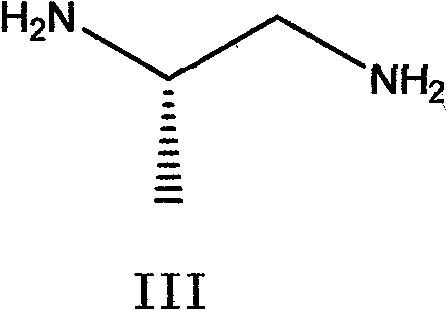
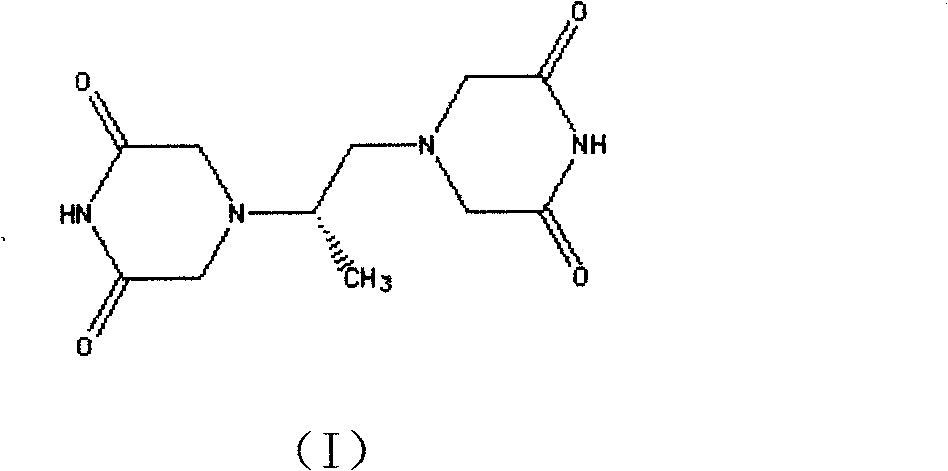
[0050] In a 2000ml three-necked reaction flask, add 170g of the above-mentioned N,N-dichloroacetimide (II) and 2040g of methanol, add 40g of sodium ethoxide under stirring, mix and dissolve for 1 hour, and dropwise add 34g of dextrorotatory 1,2-propylenediamine And 510g of ethanol solution, heated to 50°C for 4 hours, cooled to 45°C, added 35g of sodium ethylate, heated to 80°C to continue the reaction for 4 hours, during the reaction, crystals slowly precipitated, cooled to 20 ℃, filter, wash with ethanol, filter dry, and vacuum-dry at 60-80 ℃ to obtain 160.4 g of Delazolate (I). (mp=192°-195°; Optical Rotation: [a]D+11.35°-+11.40°...
example 2
[0053] Add 1193g of chloroacetonitrile into a 3000ml three-necked reaction flask, add 3580g of sulfuric acid, stir at 25°C for 8 hours, heat up to 100°C for 1 hour, cool to 30°C, pour into 3000ml of water, stir for 2 hours, filter, and refine with ethanol. 840.3 g of N,N-dichloroacetimide (II) were obtained, mp=194-196°C.
[0054] In a 2000ml three-necked reaction flask, add 170g of the above-mentioned N,N-dichloroacetimide (II) and 510g of methanol, add 40g of potassium carbonate under stirring, mix and dissolve for 1 hour, and add 17g of dextrorotatory 1,2-propanediamine dropwise And 3400g of ethanol solution, heated up to 30°C to react for 4 hours, added 35g of sodium ethylate, heated to 80°C to continue reaction for 4 hours, during the reaction, crystals slowly precipitated, cooled to 20°C, filtered, ethanol Wash, filter dry, and vacuum-dry at 60-80° C. to obtain 156.8 g of Delazolate (I). (mp=192°-196°; Optical Rotation: [a]D+11.30°-+11.40° (c=5, DMF); total yield 74.3%)...
example 3
[0057] Add 1193g of chloroacetonitrile and 597g of phosphoric acid into a 3000ml three-necked reaction flask, stir at 25°C for 8 hours, heat up to 150°C for 1 hour, cool to 30°C, pour into 3000ml of water, stir for 2 hours, filter, and refine with ethanol. 835.5 g of N,N-dichloroacetimide (II) were obtained, mp=195-198°C.
[0058] In a 2000ml three-necked reaction flask, add 170g of the above-mentioned N,N-dichloroacetimide (II) and a mixture of 3400g of methanol and propanol (mass ratio 1:1), add 40g of sodium carbonate under stirring, stir, mix and dissolve for 1 hour , add dropwise a solution of 51g of dextrorotatory 1,2-propylenediamine and 2040g of ethanol, heat up to 80°C for 4 hours, cool to 45°C, add 35g of sodium ethylate, heat up to 80°C to continue the reaction for 4 hours after adding, During the reaction process, crystals were slowly precipitated, cooled to 20°C, filtered, washed with ethanol, filtered and dried, and vacuum-dried at 60-80°C to obtain 152.3g of Del...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com