Olprinone hydrochloric parenteral solution and method for preparing same
A technology for agricultural injections and injections, which is applied in the field of medicine, and can solve problems such as painstaking efforts, the use of pharmaceutical excipients, the weight of pharmaceutical excipients is not too suitable, and the properties of opprinone hydrochloride are not fully considered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
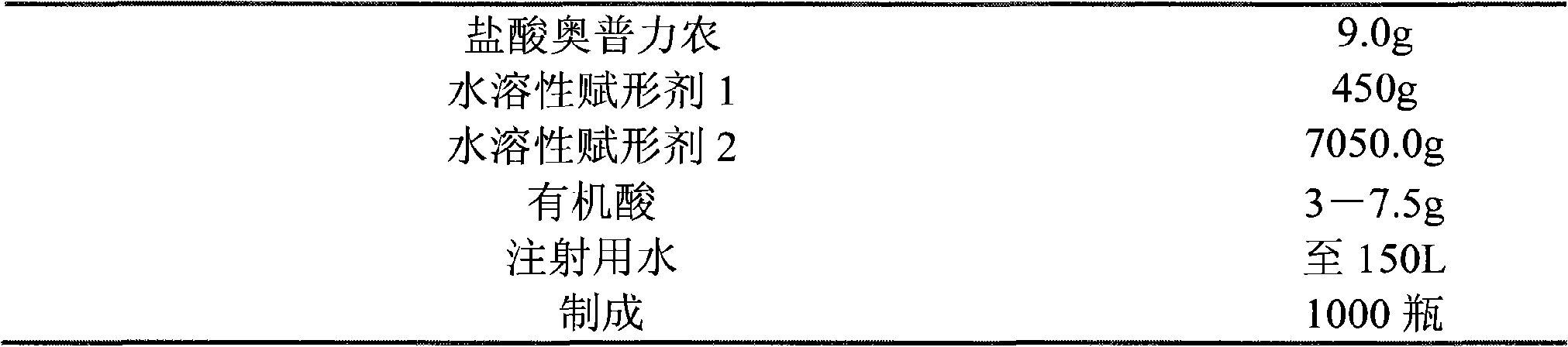
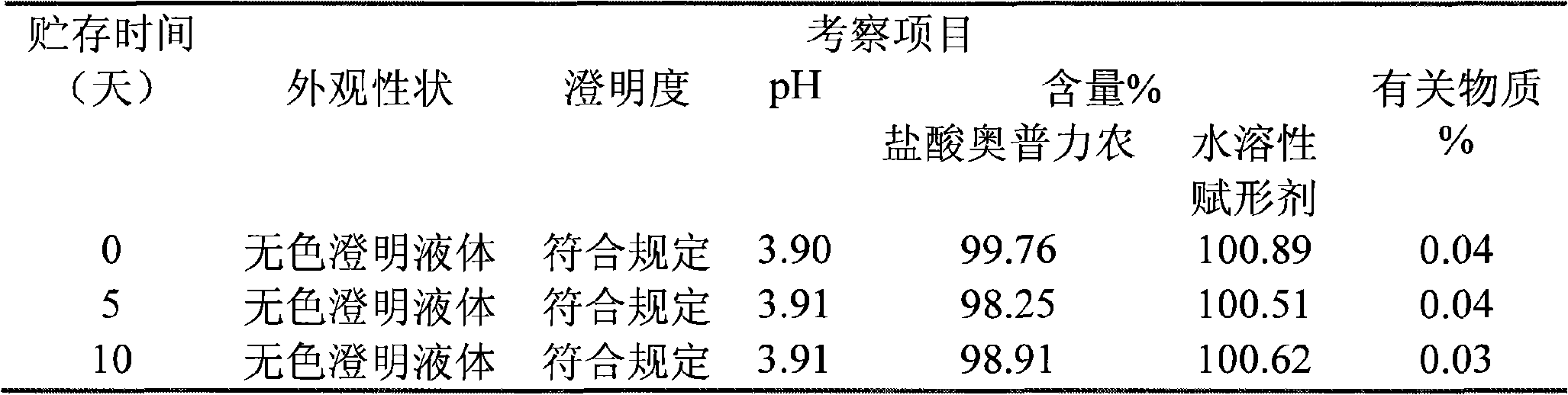
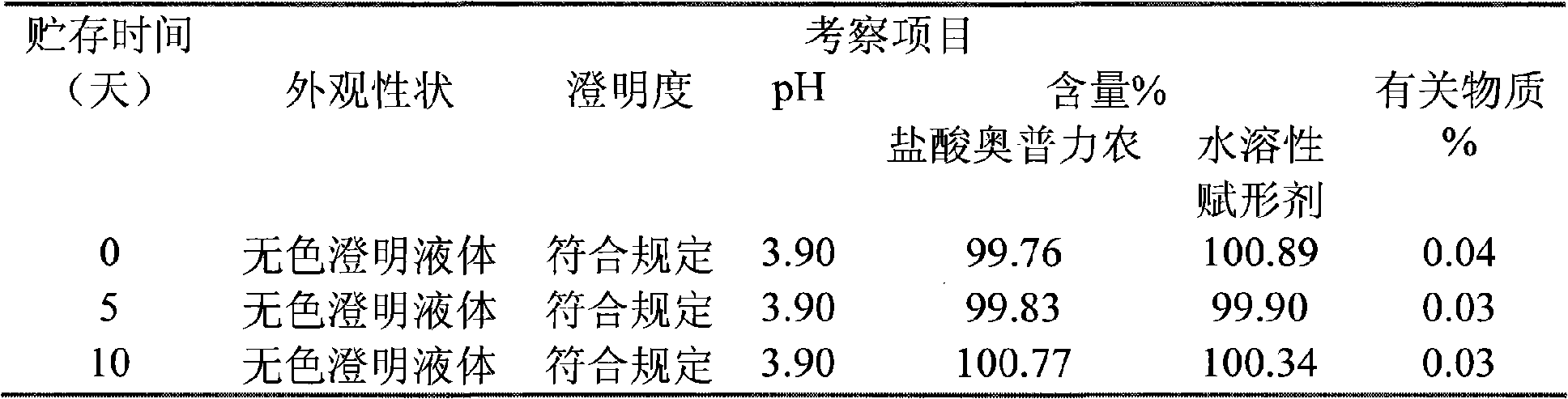
[0050] The invention relates to an injection of operprinone hydrochloride, which comprises operprinone hydrochloride and a pharmaceutical auxiliary material, wherein the pharmaceutical auxiliary material of the injection is a water-soluble excipient and an organic acid.
[0051] Among them, the pharmaceutical excipients are water-soluble excipients lactose and mannitol.
[0052] Wherein the pharmaceutical auxiliary material organic acid is citric acid.
Embodiment 2
[0054] The invention relates to an injection of operprinone hydrochloride, which comprises operprinone hydrochloride and a pharmaceutical auxiliary material, wherein the pharmaceutical auxiliary material of the injection is a water-soluble excipient and an organic acid.
[0055] Among them, the pharmaceutical excipients are water-soluble excipients glucose and mannitol.
[0056] Among them, the organic acid of pharmaceutical excipients is tartaric acid.
Embodiment 3
[0058] The invention relates to an injection of operprinone hydrochloride, which comprises operprinone hydrochloride and a pharmaceutical auxiliary material, wherein the pharmaceutical auxiliary material of the injection is a water-soluble excipient and an organic acid.
[0059] Among them, the pharmaceutical excipients are water-soluble excipients lactose and xylitol.
[0060] Wherein the pharmaceutical excipient organic acid is stearic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com