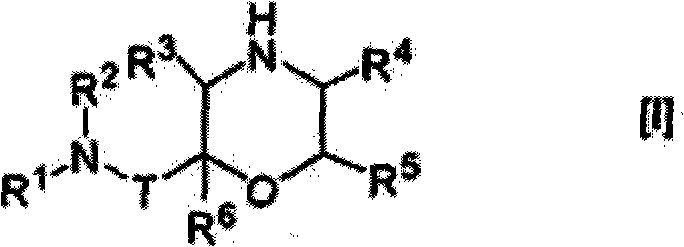
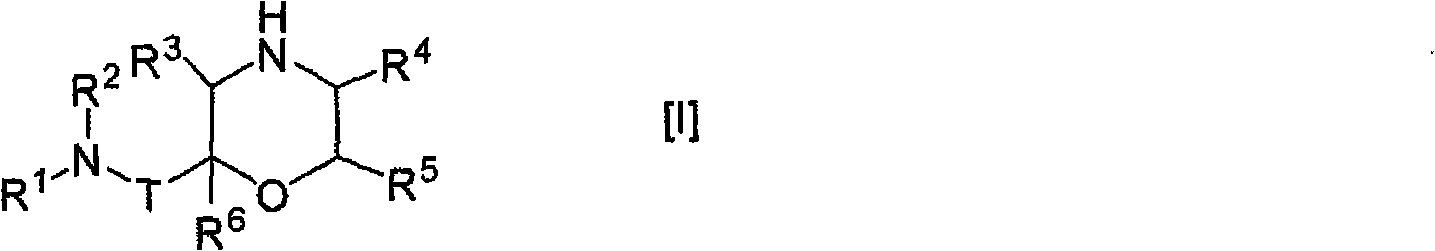Morpholine derivative
A technology of morpholine derivatives and alkyl groups, which is applied in the field of medicine and can solve the problems of not disclosing details of morpholine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0280] 【Chemical 21】
[0281]
[0282] (2R)-2-{Cyclopropyl[4-methoxy-3-(3-methoxypropoxy)benzyl]carbamoyl}ylmorpholine-4-carboxylic acid tert-butyl ester (reference example Compound 8 (368 mg) was dissolved in chloroform (4 ml), cooled in an ice bath, 4N hydrogen chloride / dioxane (1 ml) was added, and stirred at room temperature for 2 hours. Cool in an ice bath, add water, and separate the organic phase. Add saturated aqueous sodium bicarbonate solution to the aqueous phase to adjust the pH value to 9, then extract with chloroform. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by NH-silica gel column (column solvent: n-hexane / ethyl acetate=1 / 1→ethyl acetate) to obtain colorless oil (2R)-N-cyclopropyl-N-[4-methyl Oxy-3-(3-methoxypropoxy)benzyl]morpholine-2-carboxamide (230 mg). The obtained compound was dissolved in chloroform (1 ml), cooled with ice water, 4N hydr...
Embodiment 2
[0285] 【Chemical 22】
[0286]
[0287] (2R)-2-{[4-Benzyloxy-3-(3-methoxypropoxy)benzyl]cyclopropylcarbamoyl}morpholine-4-carboxylic acid tert-butyl ester (reference The compound of Example 9 (217 mg) was dissolved in chloroform (1 ml), cooled in an ice bath, 4N hydrogen chloride / dioxane (1 ml) was added, and then stirred at room temperature for 2 hours. After concentration under reduced pressure, water (1ml) and diethyl ether (2ml) were added to the residue and stirred gently, and the diethyl ether was removed by decantation. Then obtain colorless powder (2R)-N-[4-(benzyloxy)-3-(3-methoxypropoxy)benzyl]-N-cyclopropylmorpholine-2 by lyophilization - Formamide hydrochloride (166 mg).
[0288] APCI-MS m / z: 455[M+H] +
Embodiment 3
[0290] 【Chemical 23】
[0291]
[0292] (2R)-2-([3-Benzyloxy-5-(3-methoxypropoxy)benzyl]cyclopropylcarbamate)morpholine-4-carboxylic acid tert-butyl ester (reference example 10 Compound 150 mg) was obtained in the same manner as Example 2 to obtain colorless viscous oil (2R)-N-[3-(benzyl)-5-(3-methoxypropoxy)phenoxy]-N-cyclopropane Methyl-morpholine-2-carboxamide hydrochloride (107 mg).
[0293] APCI-MS m / z: 455[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com