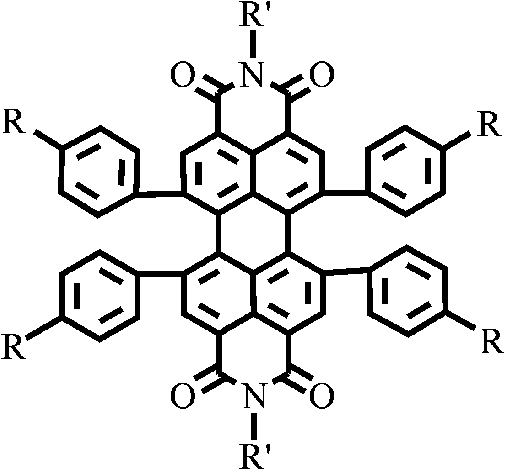
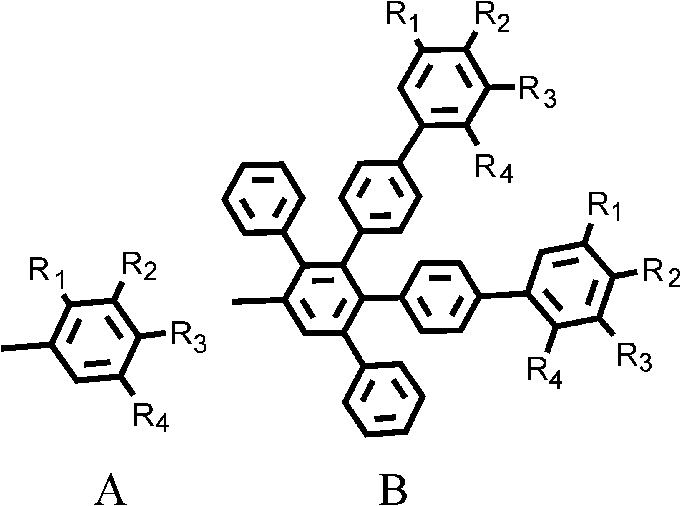
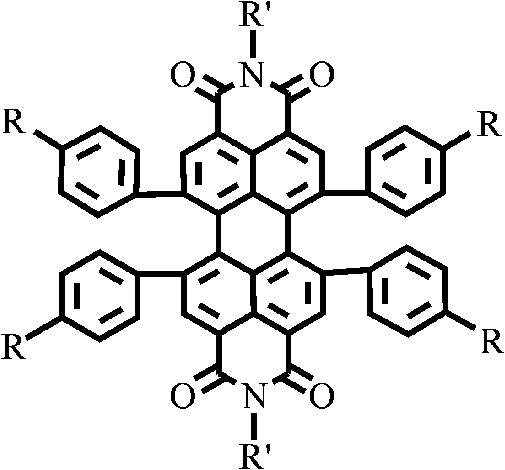
1, 6, 7, 12-tetraphenyl perylene bisimide derivant and preparation method thereof
A technology of tetraphenyl perylene bisimide and perylene bisimide, which is applied in the field of organic photoelectric functional materials, and achieves the effect of good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation of embodiment 1 compound 6
[0019]
[0020] Add 1g (1mmol) 1, 1g (2.5mmol) bis(trifluoroacetic acid) iodobenzene and 252mg elemental iodine to a 100ml two-necked flask, react at room temperature in carbon tetrachloride for 3h, separate by column chromatography to obtain 2(840mg, produce rate of 60%). MS: m / z: 1422 ([M] + ).
[0021]
[0022] Add 1.0g (0.7mmol) intermediate 2, 59mg (0.084mmol) [PdCl2 (PPh3) 2], 66.7mg (0.35mmol) CuI and 91.8mg (0.35mmol) PPh to the 50ml two-necked bottle 3 , then add 20ml of triethylamine and 10ml of THF, add 0.45ml of trimethylsilylacetylene under the protection of nitrogen, stir at room temperature for 24h, then pour into dichloromethane, filter, and obtain pure product by column chromatography, and then this pure 800mg (0.63mmol) of the product was mixed with 186.5mg (5.04mmol) of ammonium fluoride and 24.7mg (0.09mmol) of n-butylammonium fluoride, reacted in tetrahydrofuran solution at room temperature for 3...
Embodiment 2
[0027] The preparation of embodiment 2 compound 8
[0028]
[0029] 500mg (0.49mmol) of intermediate 3 and 2.76g (3mmol) of 7 were reacted in o-xylene at 160°C for 50h under nitrogen protection. Then cooled to room temperature, the solvent was removed under reduced pressure, and the target compound was separated by column chromatography 1.94g (85%) MS (MALDI-TOF): m / z: 4657.5 ([M+H] + ).
Embodiment 3
[0030] The preparation of embodiment 3 compound 10
[0031]
[0032] 100mg (0.1mmol) of intermediate 3 and 446.4mg (0.6mmol) of 9 were reacted in o-xylene at 170°C for 48h under nitrogen protection. Then it was cooled to room temperature, the solvent was removed under reduced pressure, and 271.5 mg (70%) of the target compound was separated by column chromatography. MS (MALDI-TOF): m / z: 3879 ([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com