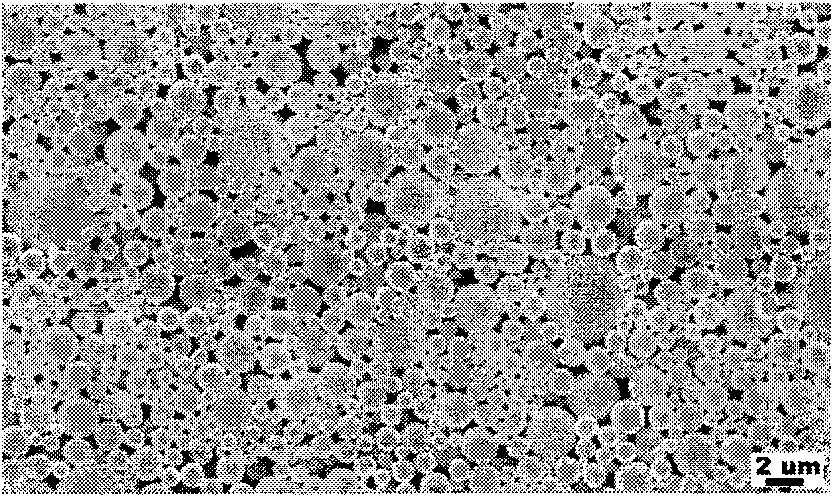
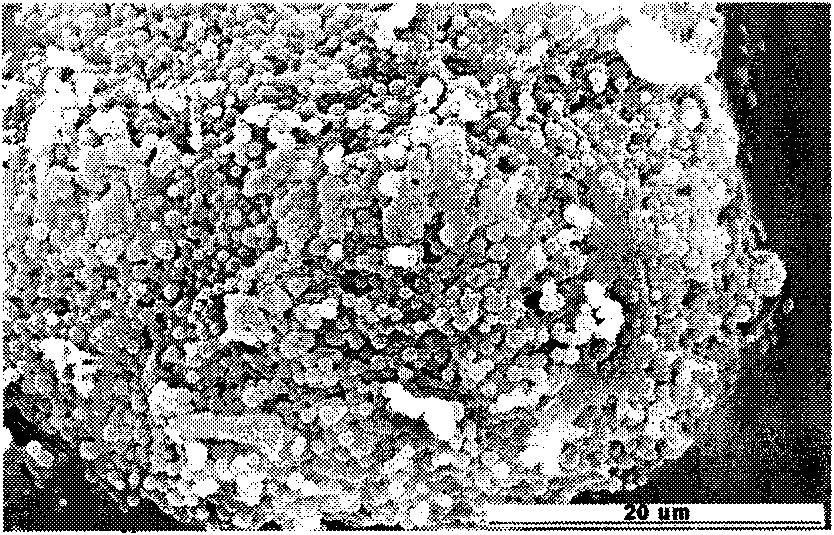
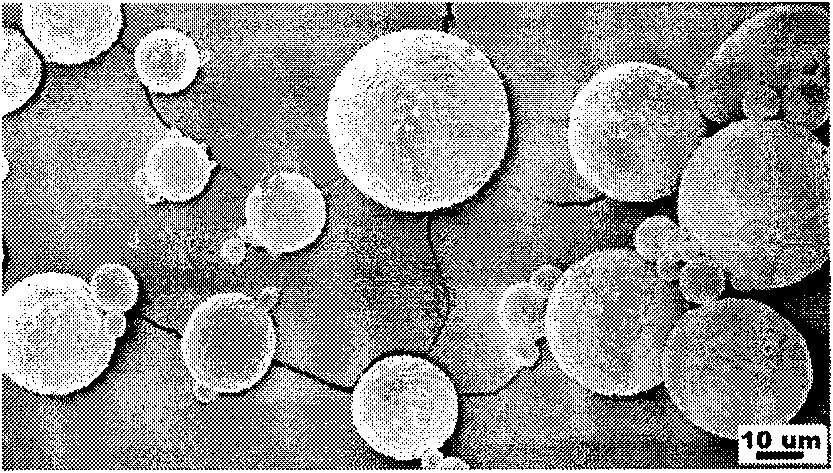Dual drug-loading composite microsphere and preparation method thereof
A technology of composite microspheres and double drug loading, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problem of excessive drug release and mechanical loss, and is not suitable for lipophilic drugs. Carriers, quality control and other issues, to achieve good biocompatibility and degradability, good biocompatibility, and increase compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation I of composite microsphere of the present invention
[0045] 1) Prepare PLGA internal microspheres Take 0.4mL of 5% (w / v) bovine serum albumin (BSA) aqueous solution and place in 2mL of PLGA containing 10% (w / v) (molecular weight 50KDa, LA: GA mole percentage is 75:25) in DCM solution, ultrasonic emulsification at 60w for 10 seconds, with an interval of 10 seconds, and repeat the "ultrasonic-interval" process 5 times to obtain an inner emulsion (w / o); in 30mL deionized water, add 0.3mL Tween 80, mechanically stirred until uniformly dispersed, and the outer water phase was obtained; the inner emulsion was added to the outer water phase, ultrasonically emulsified, and emulsified with an ultrasonic intensity of 400w for 10 seconds, with an interval of 10 seconds, and repeated 5 times to obtain a complex emulsion (w / o / w); the solution was stirred at a medium speed for 2 hours until the DCM was volatilized, and left to stand overnight. After c...
Embodiment 2
[0048] The preparation II of embodiment 2 composite microspheres of the present invention
[0049] 1) Preparation of PLGA internal microspheres
[0050] Get 0.2 mL of 5% bovine serum albumin (BSA) aqueous solution and place it in 2 mL of DCM solution containing 5% (w / v) PLGA (molecular weight 5KDa, LA: GA molar percentage is 75: 25), with 100w Intensity ultrasonic emulsification for 10 seconds, with an interval of 10 seconds, and repeat the "ultrasound-interval" process 5 times to obtain an inner emulsion (w / o); add 0.3mL Tween 80 to 30mL deionized water, stir mechanically until uniformly dispersed, and obtain The outer water phase; add the inner emulsion to the outer water phase, ultrasonically emulsify, emulsify with an ultrasonic intensity of 800w for 10 seconds, and repeat 3 times at intervals of 10 seconds to obtain a double emulsion (w / o / w); stir the liquid at a medium speed Wait for 2 hours for the DCM to volatilize, and let stand overnight. After centrifugation, wash...
Embodiment 3
[0052] Embodiment 3 Preparation III of composite microspheres of the present invention
[0053] 1) Preparation of PLGA internal microspheres
[0054] Take 0.2 mL of 5% gentamycin aqueous solution and place it in 2 mL of DCM solution containing 5% (w / v) PLGA (molecular weight 100 KDa, LA: GA mole percentage is 50: 50), and ultrasonically emulsify at 40 w 10 seconds, with an interval of 10 seconds, repeat the "ultrasound-interval" process 5 times to obtain the inner emulsion (w / o); add 0.3mL Tween 80 to 30mL deionized water, stir mechanically until the dispersion is uniform, and obtain the outer aqueous phase Add the inner emulsion to the outer water phase, supersonic emulsify, emulsify for 10 seconds with an ultrasonic intensity of 400w, and repeat 3 times at an interval of 10 seconds to obtain a double emulsion (w / o / w); stir the liquid at a medium speed for 2 hours and wait The DCM was evaporated and left to stand overnight. After centrifugation, wash with water three times ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com