Dihydrochalcone-like compound contained in tobacco and preparation method and application thereof
A compound, tobacco technology, applied in the field of tobacco chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] - Compound preparation
[0021] Tobacco samples were collected in Yuxi, Yunnan, and the variety was K326. Sampling 2.5 kg of tobacco samples and pulverizing them to 30 meshes, ultrasonically extracting 3 times with 70% acetone, combining the extracts, filtering, concentrating the extracts under reduced pressure to a small volume, filtering out the precipitate after standing, and using ethyl acetate for the filtrate The extraction was divided into 3 times, and the ethyl acetate extracts were combined and concentrated into an extract to obtain 58.4 g of extract. After dissolving the extract with an appropriate amount of chloroform, mix the sample with 80 g of crude silica gel (80-100 mesh), pack 1.5 kg of silica gel (160-200 mesh) into a column and perform silica gel column chromatography, chloroform: acetone (1:0 → 0:1) gradient Elution, TLC monitoring combined the same fractions to obtain 8 fractions (pure chloroform, chloroform-acetone 20:1, chloroform-acetone 9:1, ch...
Embodiment 2
[0023] - identification of compounds
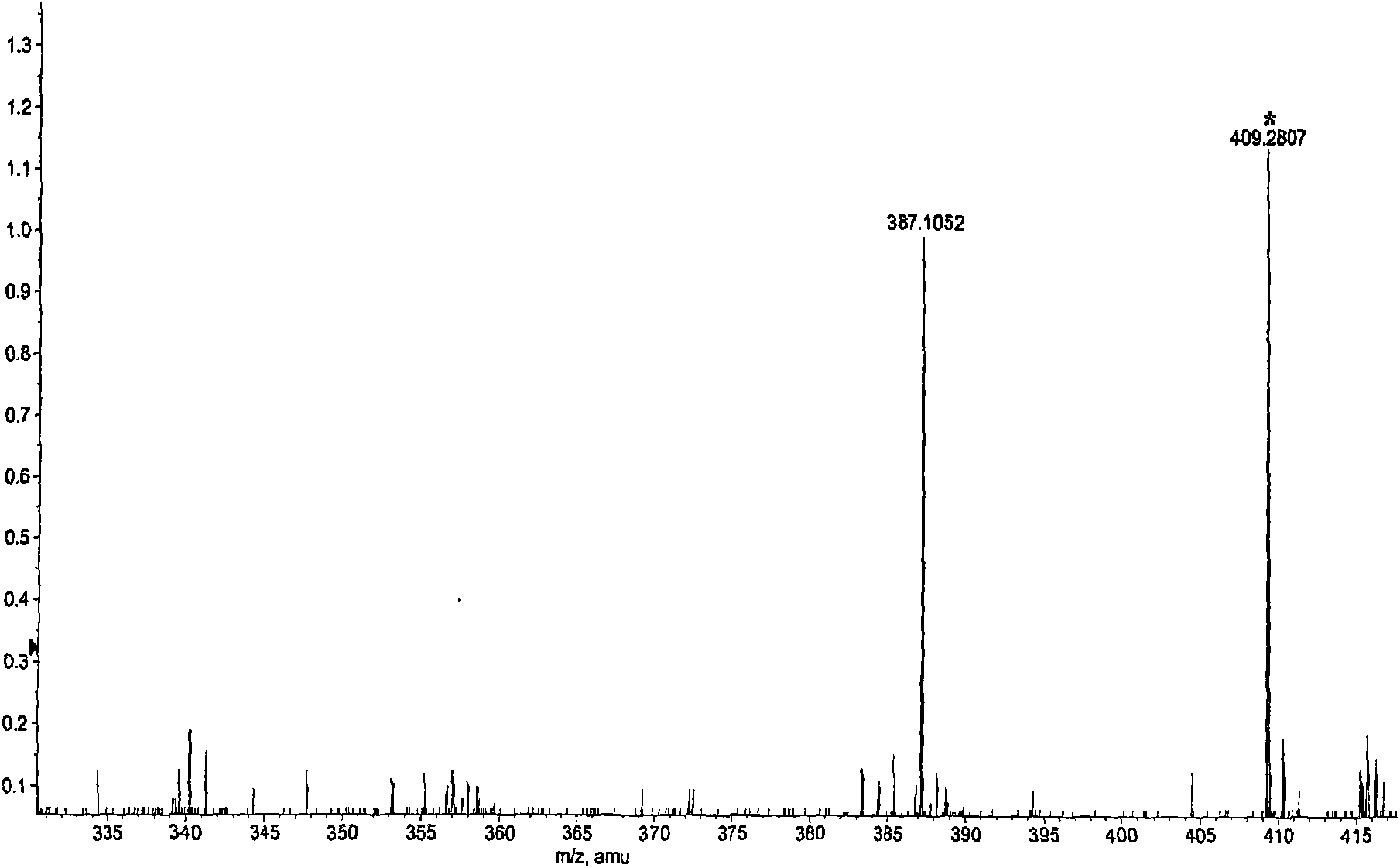
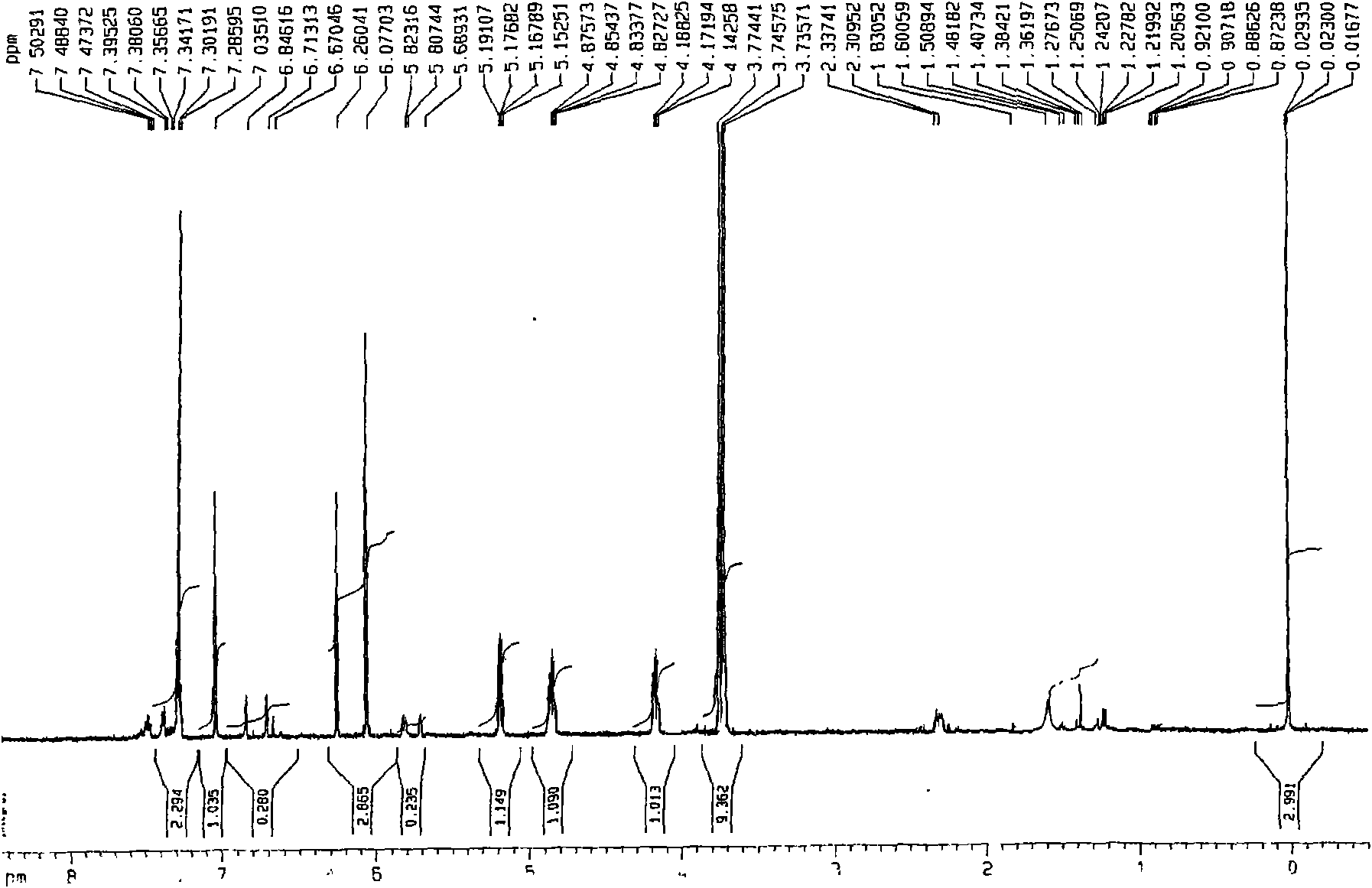
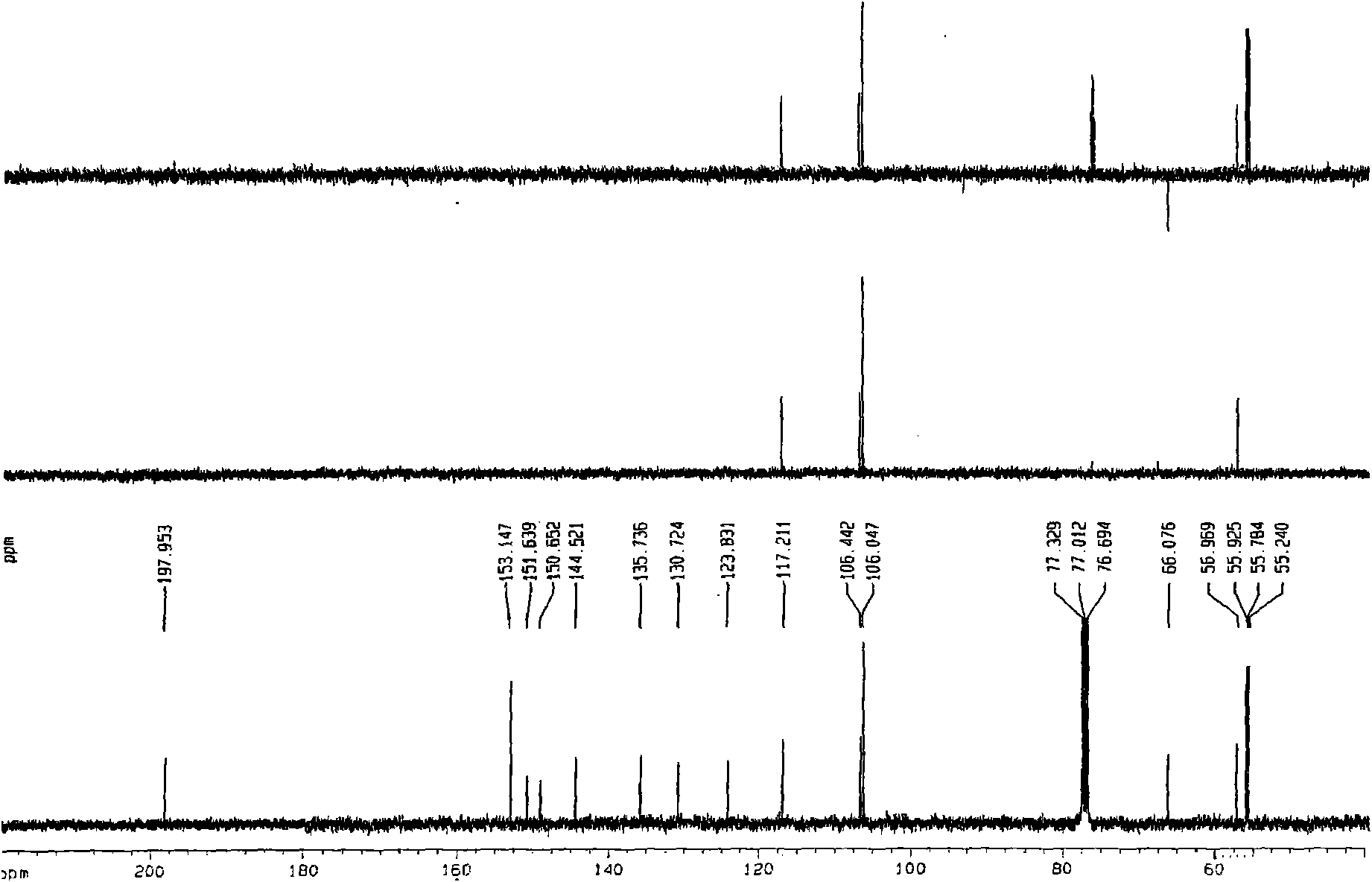
[0024] This patent compound is light yellow powder; Ultraviolet spectrum (solvent is methanol), λ max (logε)λ max (log ε)285(4.08), 234(4.41), 210(4.62)nm; Infrared Spectrum (Potassium Bromide Tablet)v max 3547, 3502, 2897, 2871, 1748, 1672, 1648, 1615, 1430, 1386, 1128, 1052, 918cm -1 ; HRES IMS (Fig.-1) gives quasi-molecular ion peak m / z 387.1052[M+Na] + (calculated value 387.1056). combine 1 H and 13 C NMR spectrum gives a molecular formula C 18 h 20 o 8 , with an unsaturation of 9. from 1 H and 13 CNMR spectrum (Figure-2 and Figure-3, see Table-1 for data attribution) signal can be seen from 1 H and 13 CNMR spectrum can see that there are 2 benzene rings in the compound (including 4 double-bonded methine carbons, δ C 106.4, 117.2, 106.0, 106.0); in addition, there is a carbonyl group (δ C 198.0), 1 methine (δ c 56.9), 1 oxidized methylene (δ C 66.1), 3 methoxy groups (δ C 55.2, 55.8, 55.8), so it can be speculated t...
Embodiment 3
[0028] ——Detection of compound antioxidant activity
[0029] The antioxidant activity is expressed by the ability to scavenge DPPH free radicals; the activity of scavenging lipid free radicals DPPH is determined with 50 μg / mL as the initial screening concentration. Take a costar 96-well plate and add freshly prepared DPPH ethanol solution (6.5×10 5 mol / L) 190 μL / well, add 10 μL / well of the sample to be tested, add 10 μL of normal saline to the blank well, mix well, seal the plate with a sealing film, keep it at room temperature for 30 minutes in the dark, and measure it on a UV2401 spectrophotometer Measure the absorbance value of each hole on the instrument, and the measurement wavelength is 517nm; the scavenging rate of the sample to the lipid free radical DPPH is calculated according to the following formula:
[0030] DPPH clearance rate (%) = (A 空白 -A 样品 ) / A 空白 ×100%
[0031] A 空白 : to the absorbance value of the white control group; A 样品 : Add the absorbance value ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com