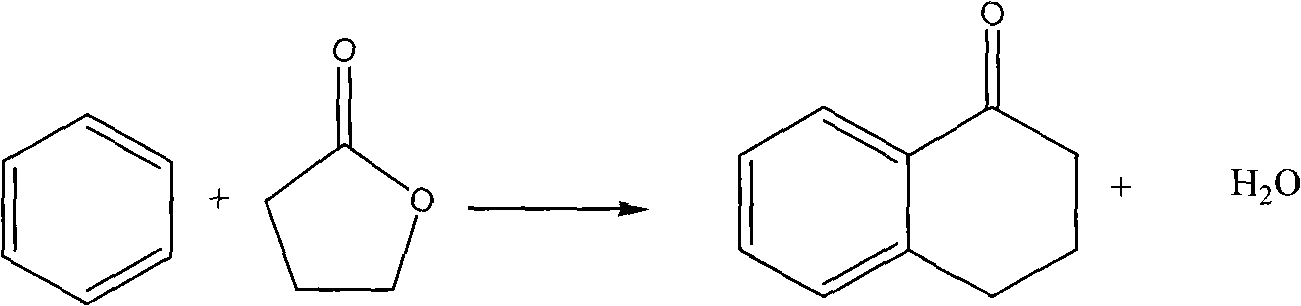
Method for synthesizing alpha-tetralone by gas solid phase reaction
A tetralone, gas-solid phase technology, applied in chemical instruments and methods, preparation of heterocyclic compounds, molecular sieve catalysts, etc., can solve problems such as difficult to recycle, unrecoverable, large amount of catalyst, etc., to improve production efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 2.2g of H-ZSM5 (Si / Al=150) (20-40 mesh) catalyst was charged into a quartz tube fixed-bed reactor with an inner diameter of 8mm and a length of 280mm, and passed N at 380°C. 2 (20ml / min) after treatment for 2h, the temperature was lowered to 240°C and kept at a constant temperature. The mixture of benzene and γ-butyrolactone (volume ratio 19 / 1) was injected with a micro injection pump at a constant flow rate (2.75mL / h, LHSV=1.1h) -1 ) into the vaporization chamber (280°C), followed by N 2 into the catalyst bed, N 2 Flow rate 20ml / min. The product condensed at the outlet of the reaction tube was collected every 20 minutes, and the content of γ-butyrolactone and α-tetralone in the product was analyzed by gas chromatography (chromatographic column: 5% FFAP / AW DMCS (60-80 mesh) )), the conversion of butyrolactone and the yield of α-tetralone were calculated. The average conversion of γ-butyrolactone was 46.5%, and the average molar yield of α-tetralone was 29.6%. The r...
Embodiment 2~4
[0027] Under other conditions and the same conditions as Example 1, the mixed solution of benzene and γ-butyrolactone (volume ratio 15 / 1), using H-Beta (S / Al=12.5) and SAPO-11 molecular sieve as catalysts, The results of the reactions under different reaction temperature, liquid space velocity and reaction time conditions are listed in Table 1 (2-4), respectively.
Embodiment 5~8
[0029] Under the same conditions as in Example 1, the mixed solution of benzene and γ-butyrolactone (volume ratio 25 / 1) was used as H-ZSM5 with different Si / Al ratios (50, 25, 19, 12.5) respectively. Catalyst at a reaction temperature of 240°C and a liquid space velocity of 1.1h -1 and the results of the reaction for 2-4 hours are listed in Table 1 (5-8), respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com