Method for preparing potassium nitrate and ammonium chloride employing double decomposition reaction
A metathesis reaction and potassium nitrate technology, applied in the direction of ammonium halide, alkali metal nitrate preparation, etc., can solve the problems of high equipment requirements, high evaporation cost, high price of sodium nitrate, etc., achieve high degree of automation, compact equipment structure, improve transmission Effect of Thermal Coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
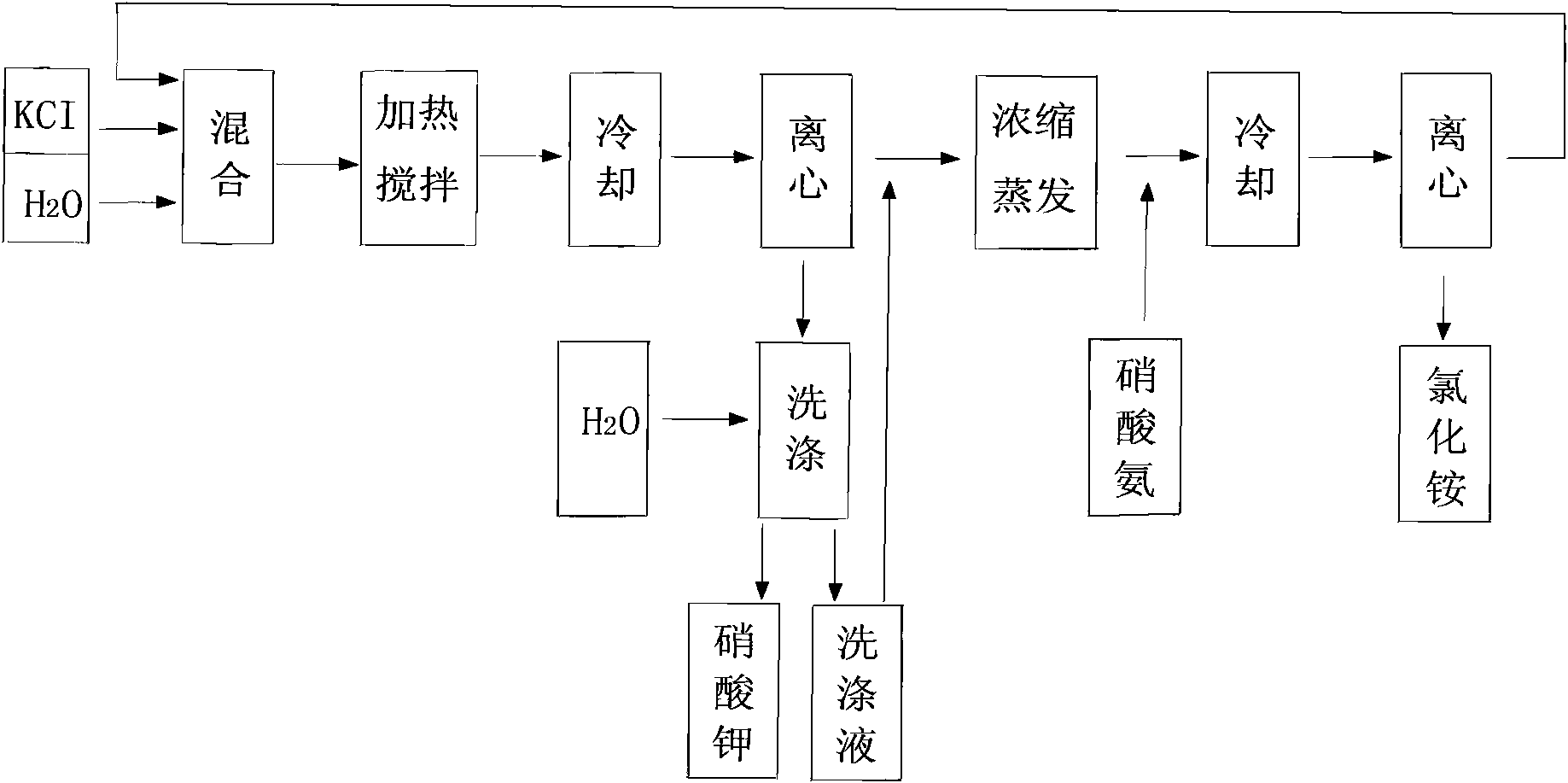
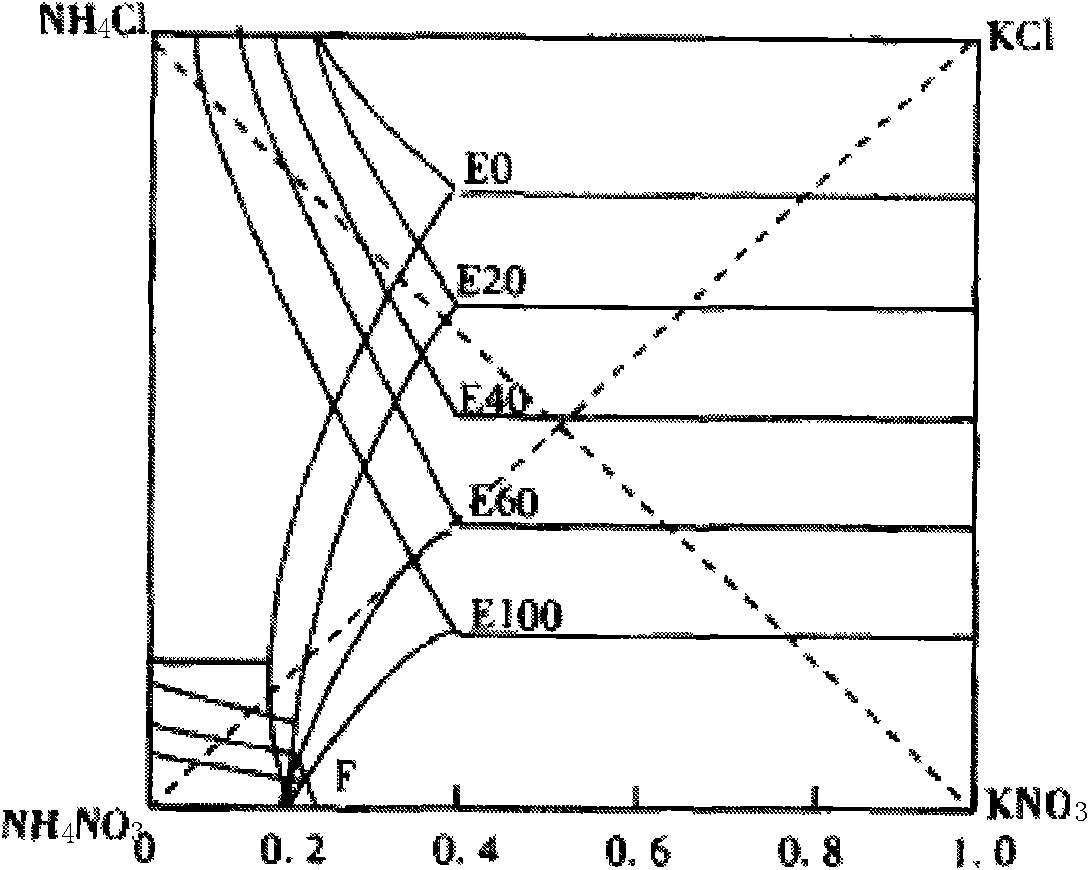
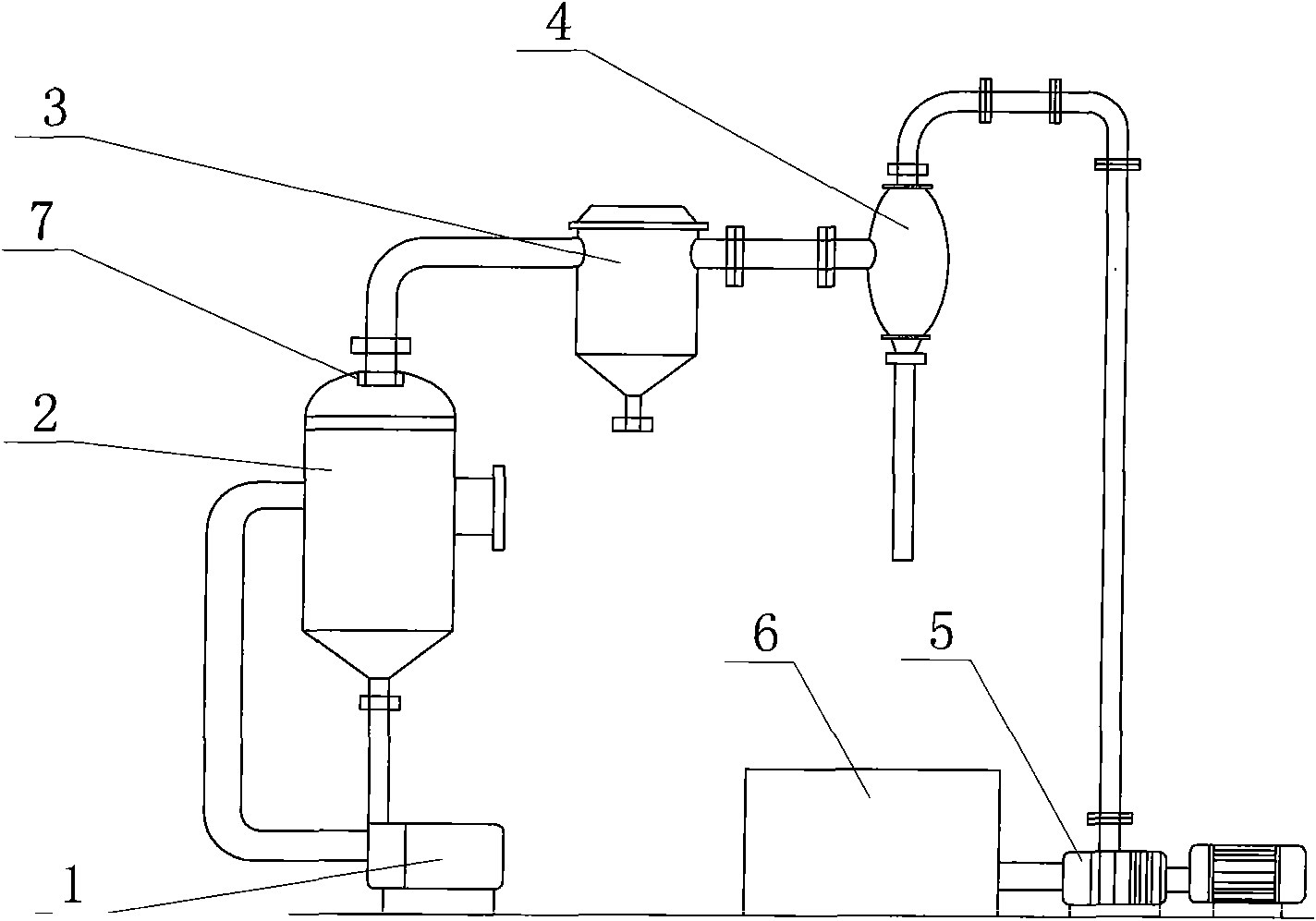
[0033] by attached figure 1 , figure 2 , image 3 , Figure 4 Shown, a kind of metathesis reaction produces the method for potassium nitrate and ammonium chloride, is raw material with ammonium nitrate and potassium chloride, carries out metathesis production potassium nitrate and by-product ammonium chloride with mother liquor circulation, and reaction formula is:
[0034] NH 4 NO 3 +KCI→KNO 3 +NH 4 CI
[0035] The technological process for producing potassium nitrate and ammonium chloride is:
[0036] A. When starting production, heat the water, add ammonium nitrate and potassium chloride, and dissolve ammonium nitrate and potassium chloride in water at a temperature of 110°C in a ratio of 1:2 between ammonium ions and chloride ions, and place in the reaction Carry out metathesis reaction in the still, form the generation material that reaches the initial co-saturation point of potassium nitrate and ammonium chloride;
[0037] B. In the reaction kettle, continue to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com