Bio-modification preparation method for improving activity of laver phycoerythrin
A phycoerythrin and biological technology, which is applied in the preparation method of peptides, chemical instruments and methods, algae/bryopeptides, etc., can solve the problems of poor stability of phycobiliproteins, and improve the value of deep processing, activity, and scavenging ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
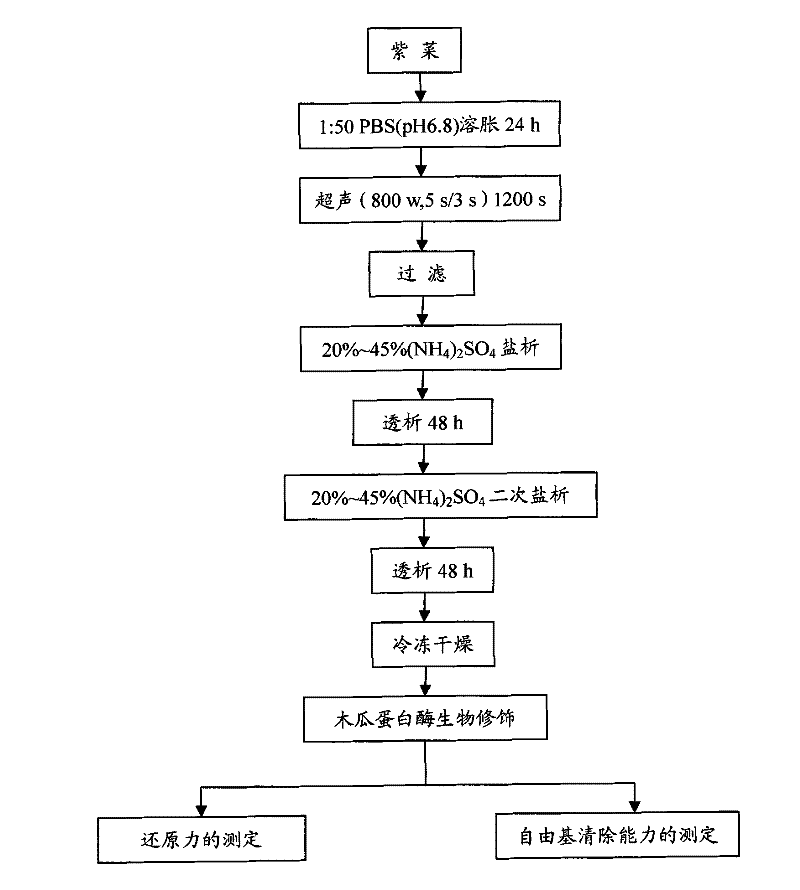
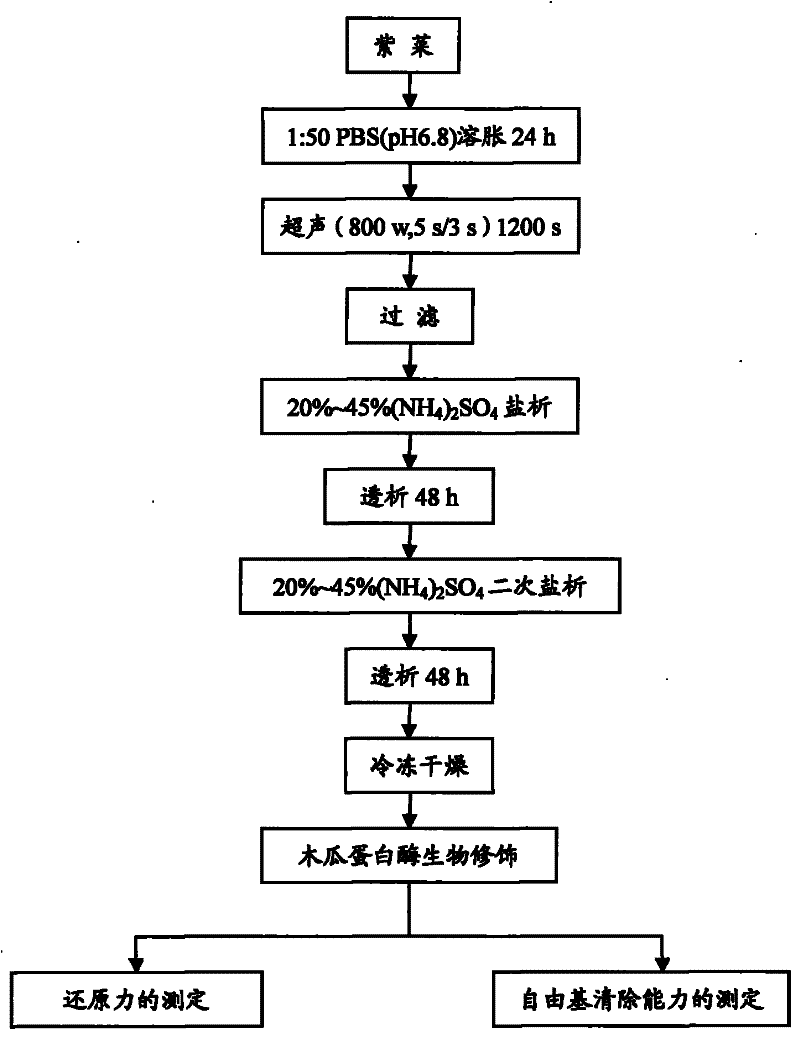
[0023] (1) Weigh 20 grams of laver powder, soak it in 1 mM PBS buffer solution with pH 6.8 at a volume ratio of 1:50 for 24 hours, and fully swell. Cell disruption was performed in an ice bath with an ultrasonic wave of 800W power for 1200s. Centrifuge at 5000rpm for 20min to obtain a crude phycoerythrin solution;
[0024] (2) Filtrate the crude phycoerythrin solution with four layers of gauze, precipitate with ammonium sulfate with a saturation of 20% to 45%, centrifuge (5000rpm, 20min), dissolve the precipitate in 1mM, pH6.8 PBS buffer solution, and run under running water Dialyze for 48 hours, until there is no white precipitate in the 20% barium chloride test, again precipitate with ammonium sulfate with a saturation of 20% to 45%, centrifuge (5000rpm, 20min), and dissolve the precipitate in 1mM, pH6.8 PBS buffer solution, running water dialysis for 48 hours, until 20% barium chloride test without white precipitate, sample freeze-drying after dialysis promptly obtains pha...
Embodiment 2
[0028](1) Weigh 20 grams of laver powder, soak it in 1 mM PBS buffer solution with pH 6.8 at a volume ratio of 1:50 for 24 hours, and fully swell. Cell disruption was performed in an ice bath with an ultrasonic wave of 800W power for 1200s. Centrifuge at 5000rpm for 20min to obtain a crude phycoerythrin solution;
[0029] (2) Precipitate with ammonium sulfate with a saturation of 20% to 45%, centrifuge (5000rpm, 20min), dissolve the precipitate in 1mM, pH6.8 PBS buffer, and dialyze for 48 hours until 20% barium chloride Check that there is no white precipitate, again precipitate with ammonium sulfate with a saturation of 20% to 45%, centrifuge (5000rpm, 20min), dissolve the precipitate in 1mM, pH6.8 PBS buffer, and dialyze with running water for 48 hours until 20 % barium chloride test has no white precipitate, and after dialysis, the sample is freeze-dried to obtain pharmaceutical grade (A 562 / A 280 >2) phycoerythrin, yield 3.12%;
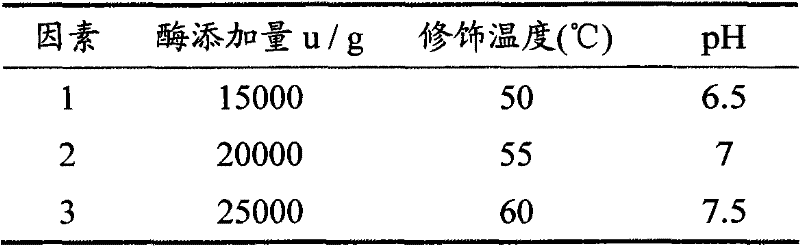
[0030] (3) Dissolve the freeze-dried p...
Embodiment 3
[0033] (1) Weigh 20 grams of laver powder, soak it in 1 mM PBS buffer solution with pH 6.8 at a volume ratio of 1:40 for 24 hours, and fully swell. Cell disruption was performed in an ice bath with an ultrasonic wave of 800W power for 1200s. Centrifuge at 5000rpm for 20min to obtain a crude phycoerythrin solution;
[0034] (2) Precipitate with ammonium sulfate with a saturation of 20% to 45%, centrifuge (5000rpm, 20min), dissolve the precipitate in 1mM, pH6.8 PBS buffer, and dialyze for 48 hours until 20% barium chloride Check that there is no white precipitate, again precipitate with ammonium sulfate with a saturation of 20% to 45%, centrifuge (5000rpm, 20min), dissolve the precipitate in 1mM, pH6.8 PBS buffer, and dialyze with running water for 48 hours until 20 % barium chloride test has no white precipitate, and after dialysis, the sample is freeze-dried to obtain pharmaceutical grade (A 562 / A 280 >2) phycoerythrin, yield 2.97%;
[0035] (3) Dissolve the freeze-dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com