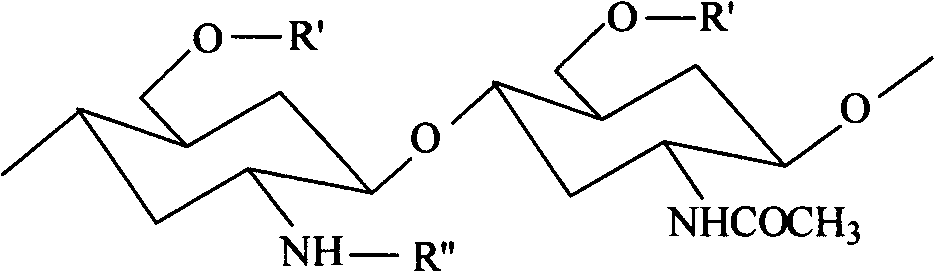
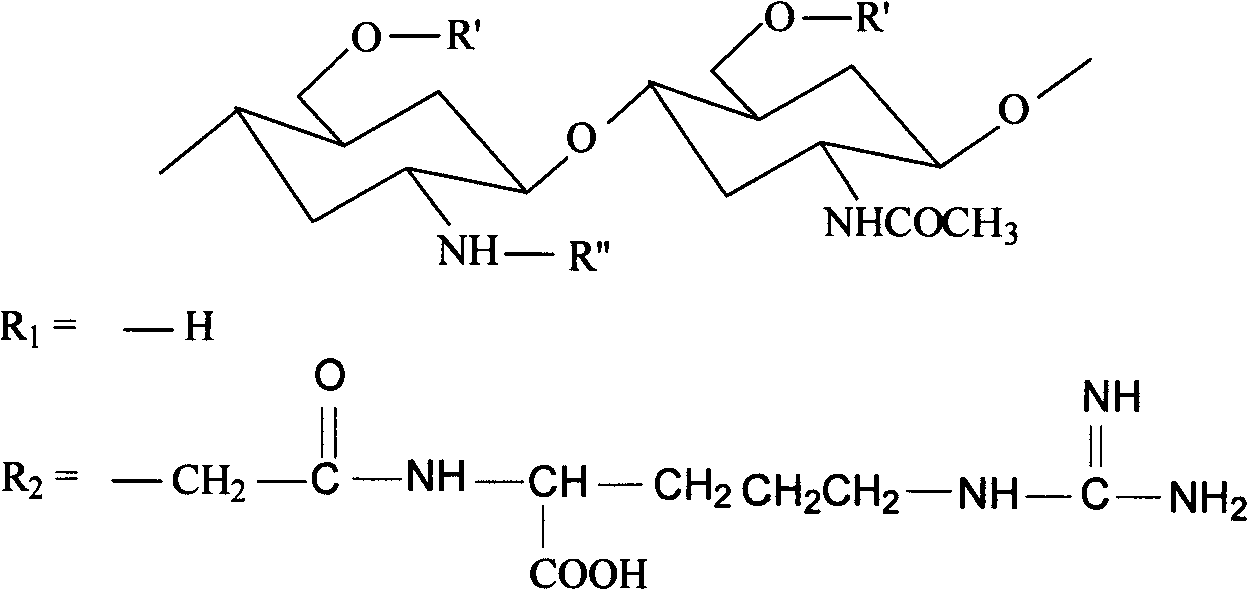
Arginine-chitosan with high degree of substitution, preparation method and application thereof
A technology of high degree of substitution and chitosan, applied in the field of biomedical materials, can solve the problem of low degree of substitution, and achieve the effects of simple preparation method, easy large-scale production and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Put 6g of chitosan into a 250ml three-neck flask, then add 50ml of isopropanol to the bottle, and stir with an electric stirrer at a speed of 400r / min for 20min at room temperature to fully mix the two. 37.5 ml of 40% sodium hydroxide solution was injected into the three-necked flask five times with an interval of 5 minutes each time. After the resulting alkaline solution was stirred for 1 hour, a total of 6 g of monochloroacetic acid was also dropped into the bottle in five batches with an interval of 5 minutes between each batch. Subsequently, the mixed system was heated up to 60° C., and reacted at this temperature for 2 hours. After the reaction was completed, after the reaction system was cooled to room temperature, 5 ml of deionized water was added thereto, and then glacial acetic acid was added to adjust the pH to 7.0, and the mixed solution was filtered. Wash and filter with 70% to 90% ethanol solution to obtain a solid. The collected solid product was dried i...
Embodiment 2
[0032] Put 6g of chitosan into a 250ml three-neck flask, then add 50ml of isopropanol to the bottle, and stir with an electric stirrer at a speed of 400r / min for 20min at room temperature to fully mix the two. 37.5 ml of 40% sodium hydroxide solution was injected into the three-necked flask five times with an interval of 5 minutes each time. After the resulting alkaline solution was stirred for 1 hour, a total of 6 g of monochloroacetic acid was also dropped into the bottle in five batches with an interval of 5 minutes between each batch. Subsequently, the temperature of the mixed system was raised to 60° C., and reacted at this temperature for 4 hours. After the reaction was completed, after the reaction system was cooled to room temperature, 5 ml of deionized water was added thereto, and then glacial acetic acid was added to adjust the pH to 7.0, and the mixed solution was filtered. Wash and filter with 70% to 90% ethanol solution to obtain a solid. The collected solid pro...
Embodiment 3
[0036] Put 6g of chitosan into a 250ml three-neck flask, then add 50ml of isopropanol to the bottle, and stir with an electric stirrer at a speed of 400r / min for 20min at room temperature to fully mix the two. 37.5 ml of 40% sodium hydroxide solution was injected into the three-necked flask five times with an interval of 5 minutes each time. After the resulting alkaline solution was stirred for 1 hour, a total of 6 g of monochloroacetic acid was also dropped into the bottle in five batches with an interval of 5 minutes between each batch. Subsequently, the temperature of the mixed system was raised to 60° C., and reacted at this temperature for 6 hours. After the reaction was completed, after the reaction system was cooled to room temperature, 5 ml of deionized water was added thereto, and then glacial acetic acid was added to adjust the pH to 7.0, and the mixed solution was filtered. Wash and filter with 70% to 90% ethanol solution to obtain a solid. The collected solid pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com