Novel muscarinic receptor antagonist and application thereof
A technology of enantiomers and optical isomers, applied in the field of new muscarinic receptor antagonists and their uses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
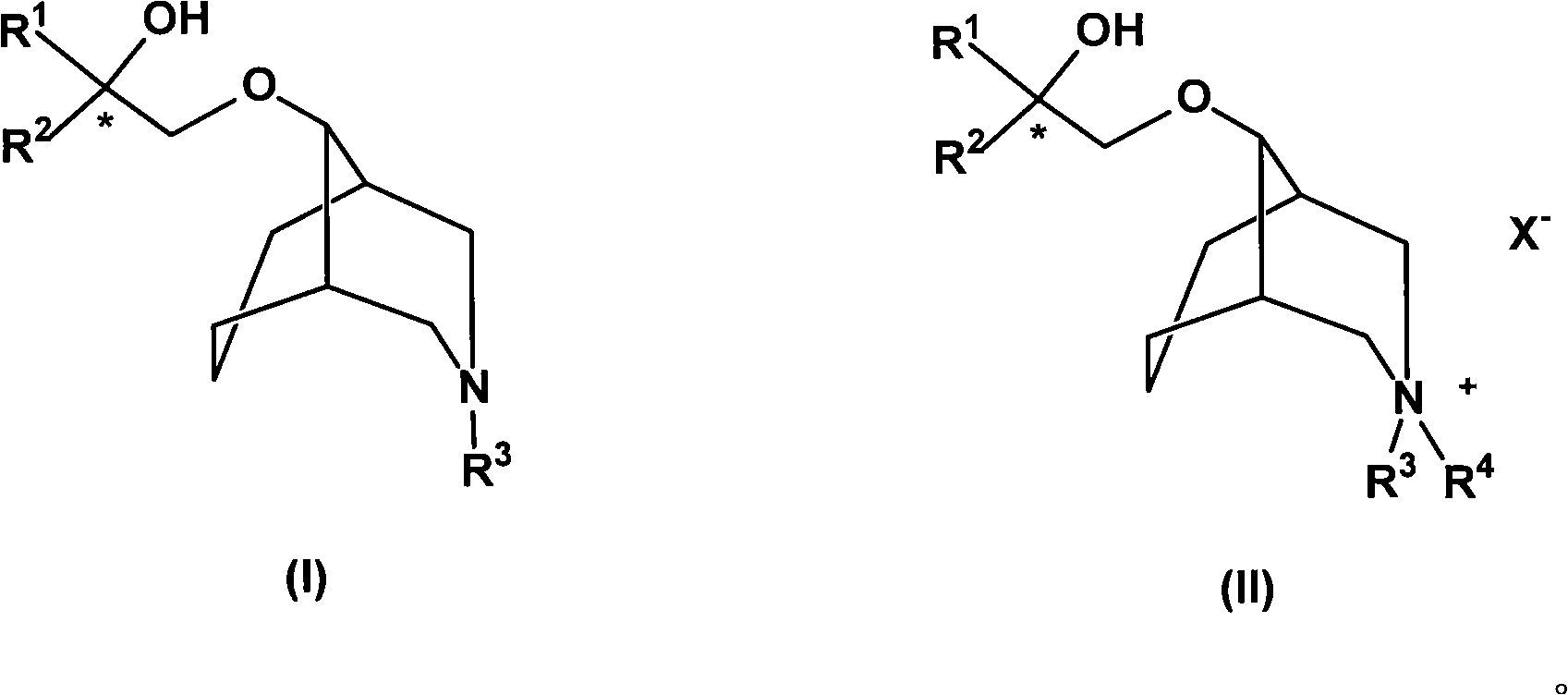
[0091] Example 1Rac-9-(2'-phenyl-2'-cyclohexyl-2'-hydroxy-ethoxy)-N-methyl-3-azabicyclo(3.3.1)nonane (Rac-1 ) preparation
[0092] Under the protection of nitrogen, put 0.6g (15mmol) NaH in a dry three-necked flask, add 10mL of anhydrous DMSO, after stirring for 5min, dropwise add N-methyl-3-azabicyclo(3.3.1) nonanol ( 2.0g (14.2mmol) in 10mL DMSO solution, stirred at 60°C for 1h. After cooling to room temperature, slowly add α-phenyl-α-cyclohexyl-1,2-oxirane 2.61g (14mmol) in 10mL DMSO solution. Stir the reaction at 50°C for 3 hours, cool, and carefully add 20 mL of water dropwise. Extract with ether, wash with water, wash the ether layer with 10% hydrochloric acid solution, and combine the water phase; Drying over sodium, distilling off the solvent to give 9-(2'-phenyl-2'-cyclohexyl-2'-hydroxy-ethoxy)-N-methyl-3-azabicyclo(3.3.1)nonane Alkane colorless liquid, 3.02g, yield 67%. 1 H-NMR: δ (ppm, CD 3 Cl), 7.44(m, 2H), 7.25(m, 3H), 5.02(s, 1H), 3.71-3.85(m, 2H), 2.91-3.05(...
Embodiment 2
[0093] Example 2Rac-9-(2'-phenyl-2'-cyclohexyl-2'-hydroxy-ethoxy)-N-methyl-3-azabicyclo(3.3.1)nonane hydrochloride ( Preparation of Rac-1·HCl)
[0094]Dissolve 2.0 g of 9-(2'-phenyl-2'-cyclohexyl-2'-hydroxy-ethoxy)-N-methyl-3-azabicyclo(3.3.1)nonane in 5 mL of ether , the ether layer was cooled, 5ml of 2N hydrochloric acid solution was added, and the solid was precipitated by stirring. The solid was collected by filtration, washed with ice water, and recrystallized from 95% ethanol to obtain 1.8 g of the title compound as a white solid, yield 81%, melting point: 189-191°C.
[0095] H-NMR: δ (ppm, CD 3 Cl), 10.87(s, 1H), 7.61(m, 2H), 7.25(m, 3H), 5.08(s, 1H), 3.63-3.80(m, 2H), 2.92-3.05(m, 4H), 2.82 (s, 3H), 2.21 (s, 1H), 2.03 (s, 1H), 2.01 (m, 2H), 1.31-1.75 (m, 12H).
Embodiment 3
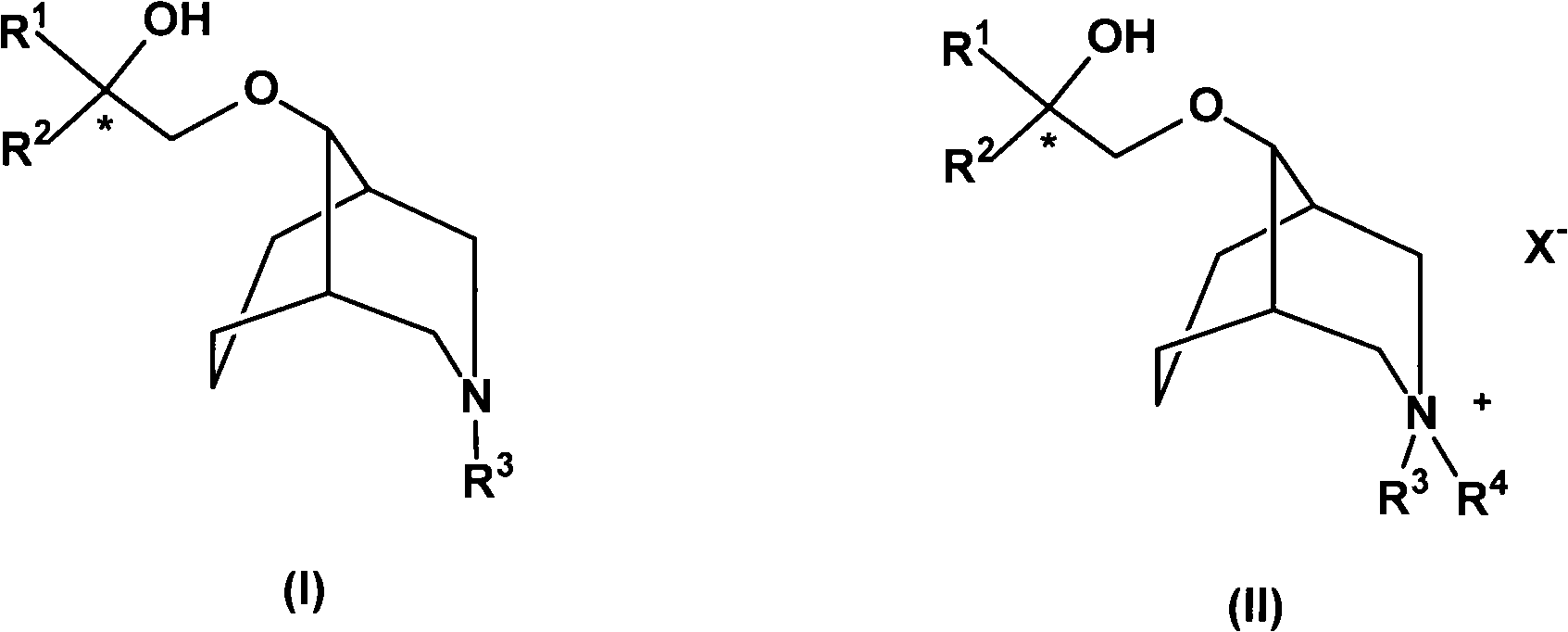
[0096] Example 3Rac-9-(2'-phenyl-2'-cyclohexyl-2'-hydroxy-ethoxy)-N,N-dimethyl-3-azabicyclo(3.3.1)nonane quaternary Amiodonium salt (Rac-1-CH 3 l) Preparation
[0097] Dissolve 1.0 g of 9-(2'-phenyl-2'-cyclohexyl-2'-hydroxy-ethoxy)-N-methyl-3-azabicyclo(3.3.1)nonane and excess iodomethane In 20mL of acetonitrile, stirred and refluxed for 5 days, TLC detected that the reaction reached equilibrium, cooled to room temperature, evaporated the solvent, the residue was washed 3 times with ethyl acetate, and recrystallized from ethanol to obtain the target compound as a yellow solid 0.86g, Yield: 61%, melting point: 161-163°C. H-NMR: δ (ppm, DMSO), 11.27 (s, 1H), 7.58 (m, 2H), 7.37 (m, 3H), 4.98 (s, 1H), 3.43-3.70 (m, 2H), 2.82- 3.10(m, 4H), 2.88(s, 3H), 2.76(s, 3H), 2.25(s, 1H), 2.06(s, 1H), 2.04(m, 2H), 1.37-1.81(m, 12H) .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com