Method for preparing low molecular weight heparin with high activity
A low-molecular-weight heparin, active technology, applied in the field of biomedicine, to achieve the effects of increased activity, reduced production costs, and huge industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, PAPS regeneration system
[0023] The concentration of components in the PAPS regeneration system is: the concentration of PNPS is 5mM, the concentration of PAP is 20μM; the dosage of AST-IV in the PAPS regeneration system is 10mg / mM PAPS. The solution prepared according to the above composition is added to the solution for preparing high-activity low-molecular-weight heparin as a sulfate group donor for the enzyme modification reaction of heparin biosynthetic enzyme 3-O-sulfatyltransferase. Such as figure 1 shown.
Embodiment 2
[0024] Example 2. Preparation of high-activity low-molecular-weight heparin using low-molecular-weight heparin Fragamine as raw material
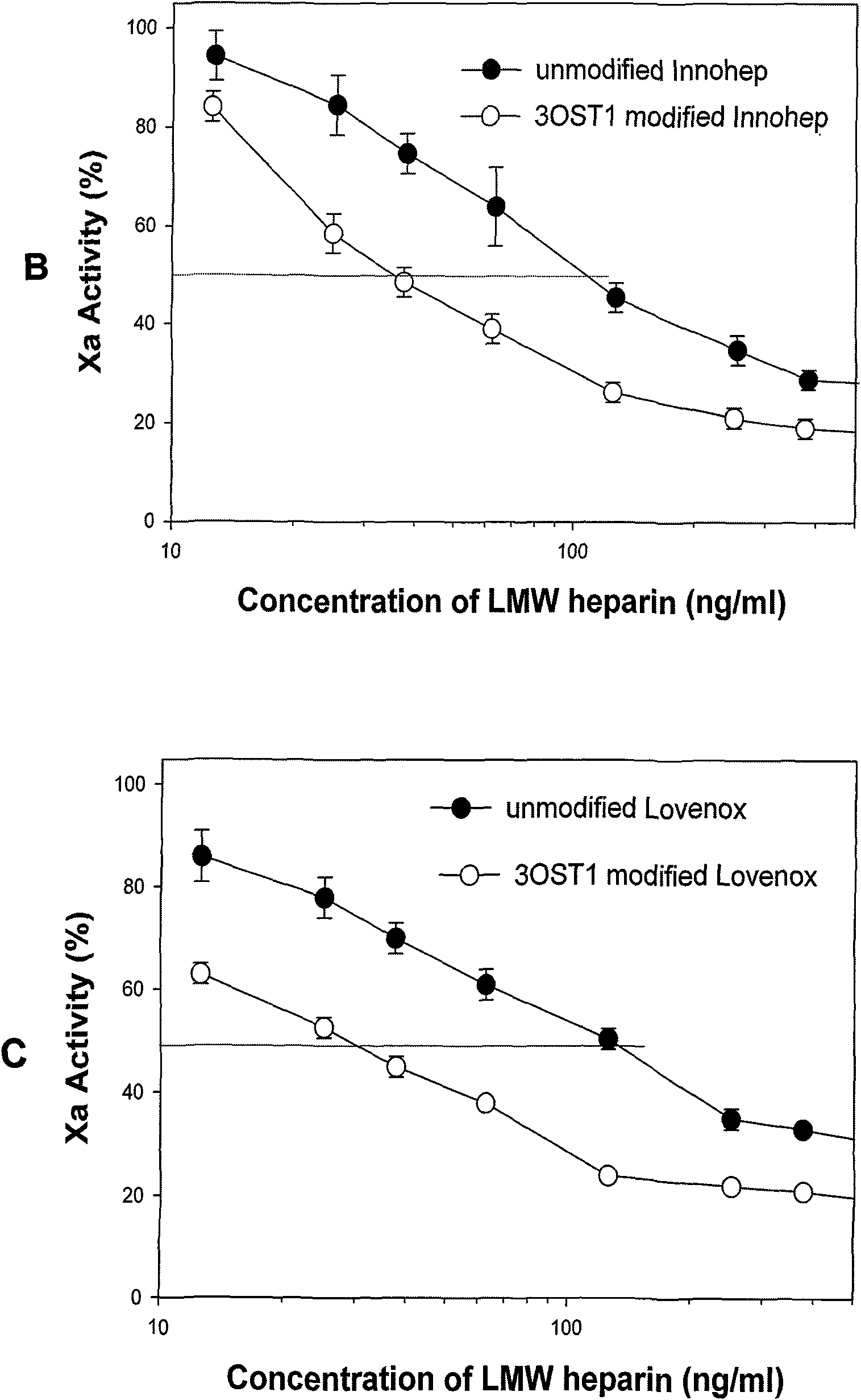
[0025] The commercial product Fragmin (Pfizer, USA, potency) was used as the raw material, and the reaction conditions were 1mg of low molecular weight heparin Fragmin in 20ml of reaction liquid and shaking (300rpm) at room temperature for 6h. The reaction solution contained 50 mM Tris-HCl (pH 7.2), 1% Triton X-100, 1% BSA, 1 mM MgCl2, 1 mM MnCl2, 1 mM PNPS, 40 μM PAP, 8 mg 3-OST-1 and 4 mg AST-IV. Heat the reaction solution at 100°C for one minute to terminate the reaction, centrifuge to obtain the supernatant, mix the supernatant with twice the volume of 2.0% NaCl aqueous solution, and load the sample on the DEAE column; sequentially use 2.0% NaCl aqueous solution and 3.0 % NaCl aqueous solution for gradient elution, and collect the eluate obtained from the second elution to obtain high-activity low-molecular-weight heparin. The resultin...
Embodiment 3
[0026] Example 3. Preparation of high-activity low-molecular-weight heparin using low-molecular-weight heparin tinzaparin as raw material
[0027]The commercial tinzaparin (DuPont, titer) was used as the raw material, and the reaction conditions were 1mg of low molecular weight heparin tinzaparin in 20ml of the reaction solution at room temperature for 6h with shaking (300rpm). The reaction solution contained 50 mM Tris-HCl (pH 7.2), 1% Triton X-100, 1% BSA, 1 mM MgCl2, 1 mM MnCl2, 1 mM PNPS, 40 μM PAP, 8 mg 3-OST-1 and 4 mg AST-IV. Heat the reaction solution at 100°C for one minute to terminate the reaction, centrifuge to obtain the supernatant, mix the supernatant with twice the volume of 2.0% NaCl aqueous solution, and load the sample on the DEAE column; sequentially use 2.0% NaCl aqueous solution and 3.0 % NaCl aqueous solution for gradient elution, and collect the eluate obtained from the second elution to obtain high-activity low-molecular-weight heparin. The resulting ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com