Novel azulene compounds and application thereof
A compound and polymer technology, applied in the field of medicine, can solve the problems of rare, few substituents of ordinary azulene compounds, limited application research, etc., and achieve a strong effect of removing free radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
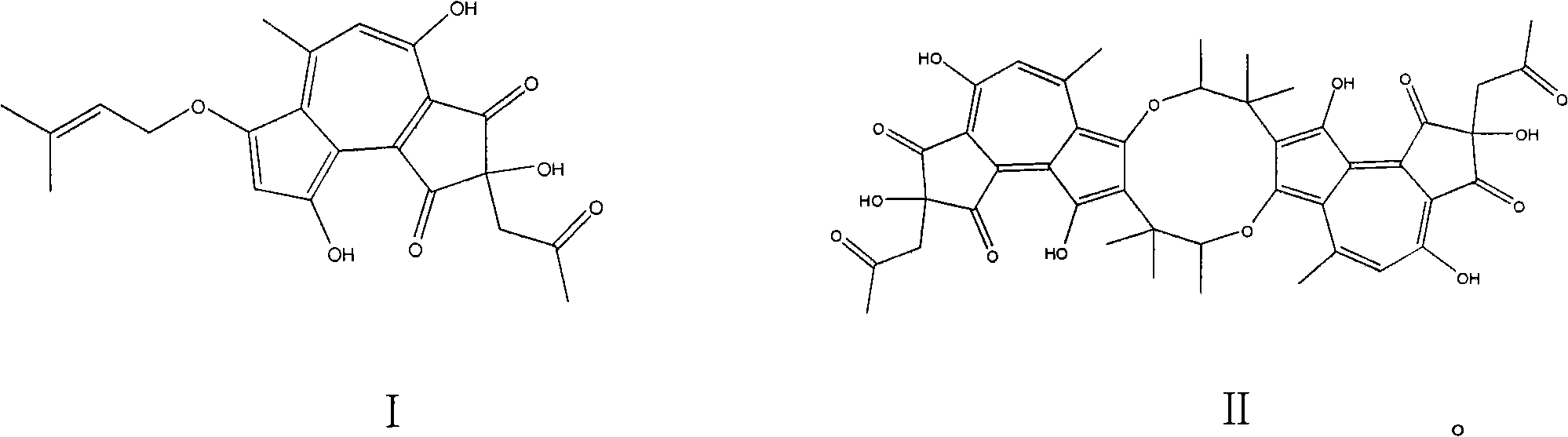
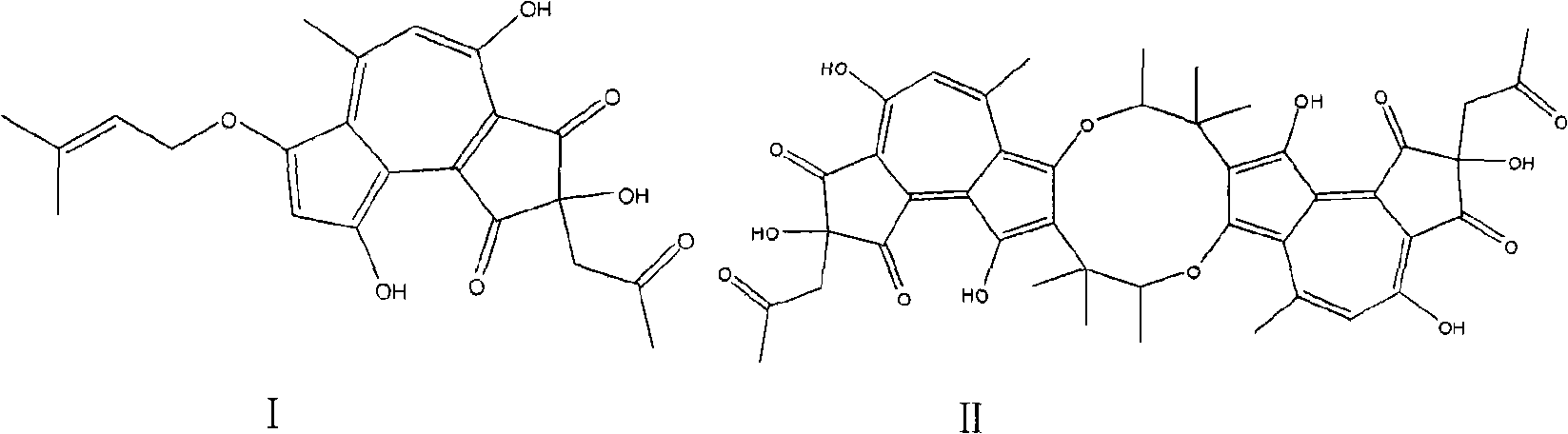
[0021] At room temperature, 300g (wet weight) mycelium was placed in a Erlenmeyer flask and extracted with 800ml 80% acetone. The extract was separated by silica gel column chromatography, and eluted with chloroform and methanol as mobile phases (100:1 , 100:3, 100:5, 100:8, 100:10, 9:1, 7:1, 5:1, 3:1), take 100:5 part. The 100:5 portion of Sephadex LH-20 was depigmented, and the volume ratio of chloroform to methanol was 1:1 as the mobile phase, and the golden yellow was used as the mobile phase. The part prepared by the above method is crudely separated by medium and low pressure, and the 70% and 80% parts are combined, and then analyzed and prepared by liquid phase preparation (methanol: water = 70%). The elution time is 40 minutes and 44 minutes, respectively. Within minutes, two compounds, compound I (3.2 mg) and compound II (4.1 mg) can be obtained, and the purity of the two compounds reached 98% or more after analysis.
Embodiment 2
[0023] At room temperature, 300g (wet weight) mycelium was placed in an Erlenmeyer flask and extracted with 800ml of 100% acetone. The extract was separated by silica gel column chromatography and eluted with chloroform and methanol as mobile phases. Take 20: 1 part. Remove the pigment from the LH-20 on the 20:1 part, use the chloroform and methanol volume ratio of 1:1 as the mobile phase, and take the lighter color and interrupt for use. The part prepared by the above method is roughly cut off at medium and low pressure, and then analyzed and prepared by liquid phase preparation (methanol: water = 70%). The elution time is 40 minutes and 44 minutes, respectively, and two compounds can be obtained. The purity of compound I (2.2mg) and compound II (3.3mg) reached more than 98% after analysis.
Embodiment 3
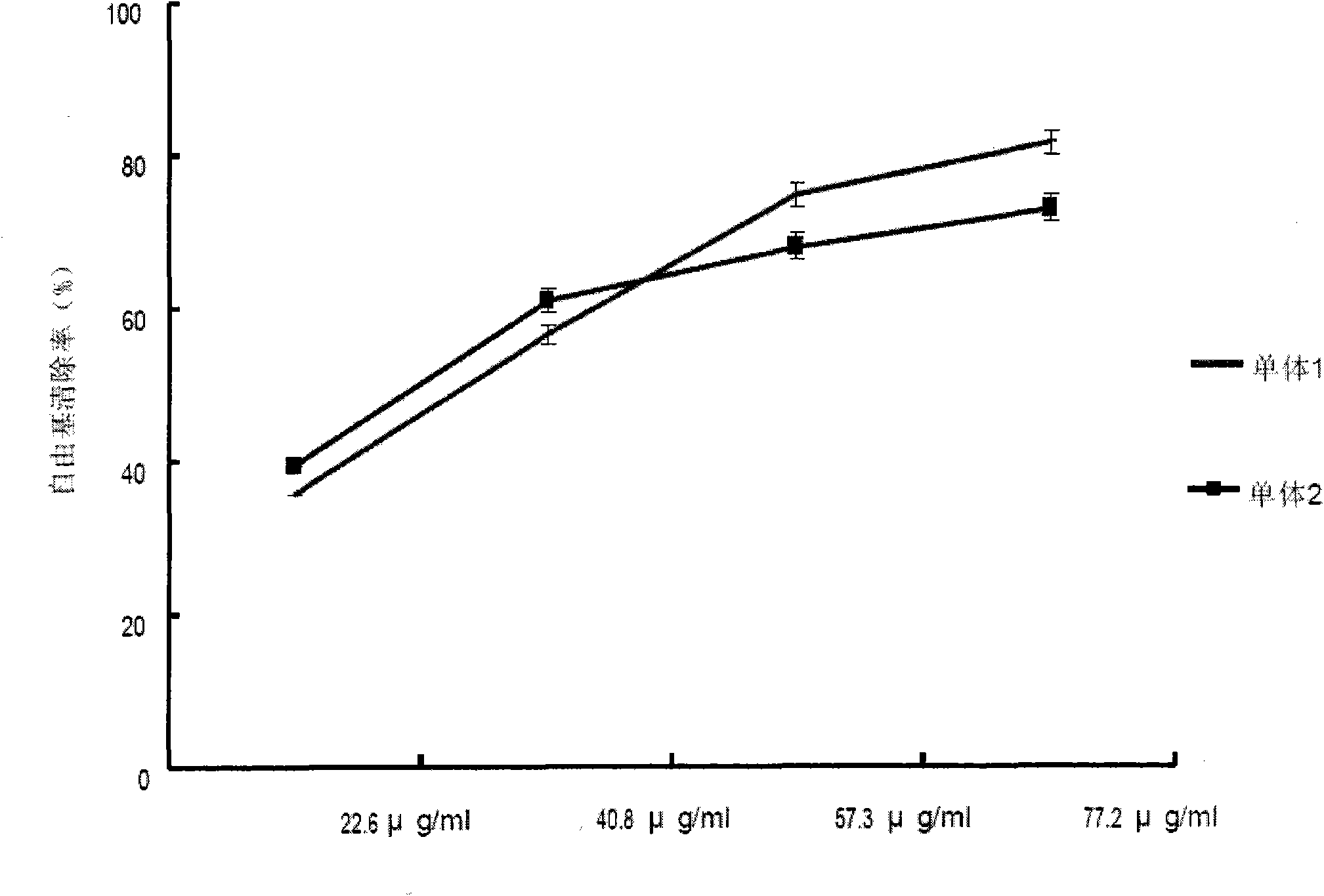
[0025] Precisely draw a small and the same volume of the sample solution into a 5mL volumetric flask, add 3mL of 31.52μg / mL DPPH solution, and dilute the volume with methanol. After standing for 1h in the dark at room temperature, the absorbance was measured at 516nm.
[0026] DPPH clearance rate calculation formula: clearance rate (%) = [1一(DPPH·) t , / (DPPH·) t=0 ]×100%
[0027] In the formula (DPPH·) t=0 Is the initial concentration of DPPH radical in the system; (DPPH·) t It is the concentration of DPPH free radicals in the solution after 1h. The sample (μg) / DPPH (μg) is plotted against the scavenging rate of DPPH free radicals.
[0028] As the dose increases, the clearance rate gradually increases, until it reaches a relatively stable level, the change trend of the clearance rate tends to slow down.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com