Functional polyfluorene compound and intermediate and application thereof
A technology of polyfluorene compounds, which is applied in the application field of functionalized polyfluorene compounds in modifying carbon nanotubes, can solve the problems of difficult control of the processing process, low solubility, low efficiency, etc., and achieve correct structure determination by instruments and preparation The method is simple and the effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, preparation functionalized polyfluorene compound intermediate
[0034] 1) Put 7.3mmol of 1-pyrene boronate, 8.1mmol of 1-(6-bromohexyloxy)-4-bromobenzene and 7.5mmol of sodium bicarbonate into 40mL of tetrahydrofuran and 15mL of water, and add 0.091mmol of catalyst Pd under nitrogen protection (PPh 3 ) 4 , reflux for 18 hours. After the reaction was completed, it was cooled, extracted, washed, dried, and separated by column chromatography to obtain 1-(4-(6-bromohexyloxy)phenyl)pyrene with a yield of 84%.
[0035] 1 H NMR (400MHz, CDCl 3, ppm): δ8.23-8.16 (m, 4H), 8.09 (s, 2H), 8.04-7.97 (m, 3H), 7.56 (d, 2H), 7.10 (d, 2H), 4.08 (t, 2H ), 3.46(t, 2H), 1.96-1.87(m, 4H), 1.60-1.55(br, 4H);
[0036] 13 C NMR (100MHz, CDCl 3 , ppm): δ158.67, 137.71, 133.60, 131.76.131.20, 130.52, 128.75, 127.87, 127.49, 126.14, 125.58, 125.19, 124.89, 124.83, 114.58, 68,03, 34.03, 29.2
[0037] Anal.Calcd.for C 28 h 25 BrO: C, 73.20; H, 5.92. Found: C, 73.47; H, 5.92...
Embodiment 2
[0042] Example 2, preparation of functionalized polyfluorene compounds
[0043] Put 0.084mmol of the polyfluorene compound intermediate prepared in Example 1, 0.085mmol of 9,9-dioctyl-2,7-diboronate-fluorene, and 3.6mmol of sodium bicarbonate into 10mL of tetrahydrofuran and 4mL of water, nitrogen protection 0.84μmol catalyst Pd(PPh 3 ) 4 , reflux for 96 hours. After the reaction is completed, cool, extract, wash, dry, and settle to obtain the functionalized polyfluorene compound (abbreviated as PFPy) represented by the formula IV, with a yield of 88%.
[0044] 1 H NMR (400MHz, CDCl 3 , ppm): δ8.19-7.91 (br, 18H), 7.84-7.83 (br, 2H), 7.7l (br, 6H), 7.54-7.51 (br, 8H), 7.31-7.28 (br, 4H), 7.05-7.02(br, 4H), 6.84-6.82(br, 4H), 4.03(m, 4H), 3.94(br, 4H), 2.03(br, 4H), 1.83(br, 8H), 1.55(br, 8H), 1.08(br, 20H), 0.81-0.79(br, 10H);
[0045] 13 C NMR (100MHz, CDCl 3 ,ppm):δ158.64,158.10,151.67,141.77,138.13,137.69,135.91,133.47,131.72,131.14,130.44,129.47,128.70,128.40,127....
Embodiment 3
[0047] Example 3, preparation of carbon nanotubes modified by functionalized polyfluorene compounds
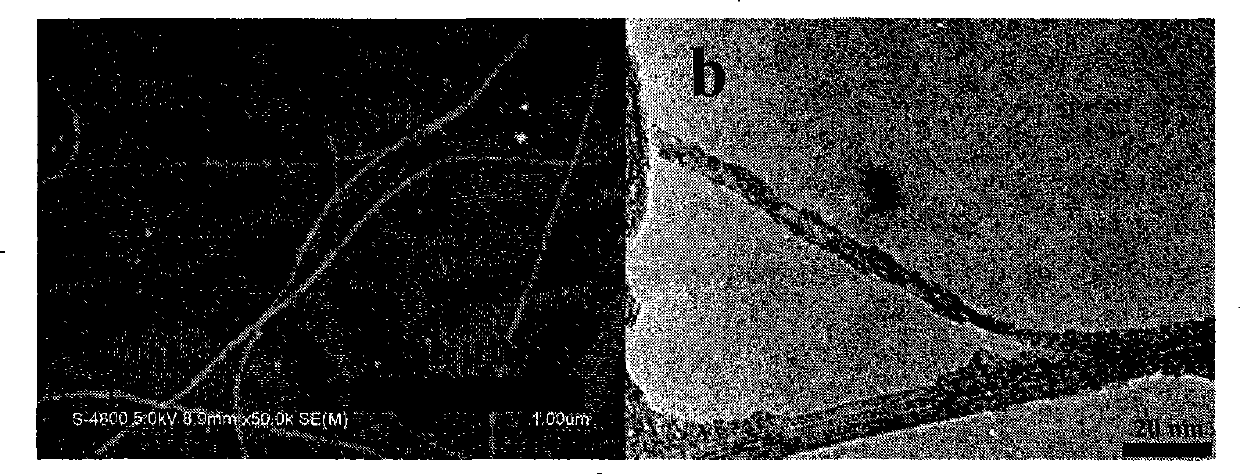
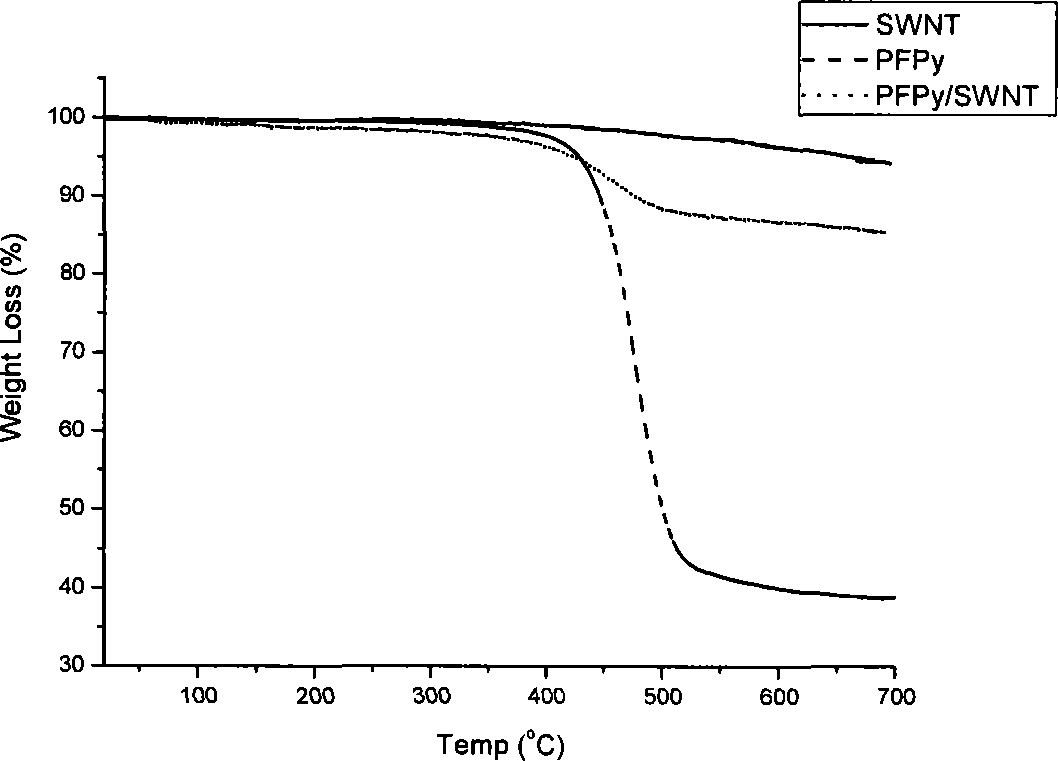
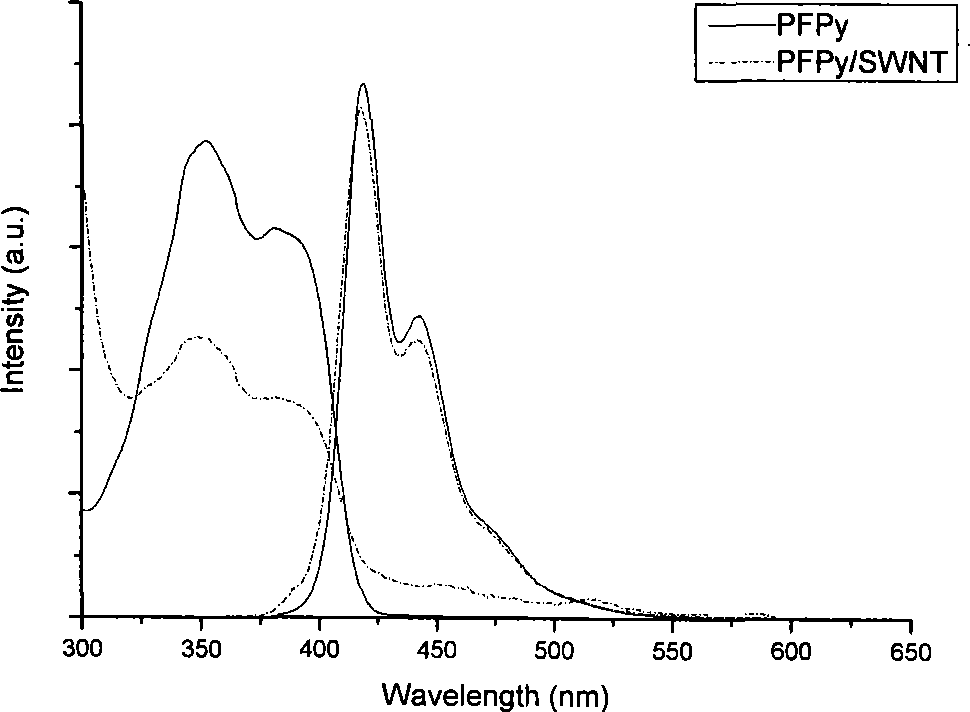
[0048] 10mg of the functionalized polyfluorene compound PFPy (M n =66000) was dissolved in 1 mL of tetrahydrofuran, and then 2 mg of starting single-armed carbon nanotubes were put into the previous solution, and the ultrasonic wave was continued for 1 hour; the dispersion was centrifuged at a speed of 3000 rpm for 10 minutes, and the supernatant black liquid was carefully collected and stored. overnight. Afterwards, the excess polymer solution was filtered off with a filter membrane with a pore size of 450 nm, and the excess polymer was washed (4×25 mL) with a large amount of tetrahydrofuran or water. After washing, the filter membrane with carbon tubes is sucked dry, and dried in a vacuum oven at 80-90°C. Finally, cut the filter membrane, carefully peel off the carbon tube from the filter membrane, and weigh it carefully. In the preparation method, the carbon nanotubes us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com