Composition containing angiotensin II receptor antagonist, statin and folic acid as well as applications thereof
An angiotensin and receptor antagonist technology, which is applied in the field of compositions and applications containing angiotensin II receptor antagonist, statin and folic acid, can solve the problem of the combination of ARB, statin lipid-lowering drugs and folic acid. and other problems, to achieve the effect of reducing ventricular diastolic insufficiency, reducing the effect, and enhancing stiffness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Protective effect of losartan + atorvastatin + folic acid on target organ in hypertensive rats with dyslipidemia
[0043] 1. Preparation of compound model animals
[0044] For SD rats, the experiment was started after 5 days of adaptive feeding. Two kidneys and one clip operation were performed to prepare 2K1C hypertensive model animals. After feeding for 6 weeks, the blood pressure of the rats was measured (measured three times to get the average value). If the blood pressure > 140mmHg, the model was considered successful, and the model group began to gavage fat emulsion (cholesterol 10g, propylene glycol 20ml, bile salt 2g, Tween-80 20ml, 1g of propylthiouracil, 20ml of peanut oil, 20g of sucrose, after dissolving, add distilled water to 200ml) 10ml / kg and free drinking water (containing 1% methionine), after 4 weeks of intragastric administration of fat emulsion (retest after 2 weeks of infusion of fat emulsion) Blood pressure at one time) The rats were ...
Embodiment 2
[0089] Example 2: Protective effect of telmisartan + simvastatin + folic acid on target organ damage in stroke-susceptible spontaneously hypertensive (SHR-SP) rats with dyslipidemia
[0090] A total of 80 male SHR-SP rats about 8 weeks old were divided into model group, telmisartan+simvastatin (0.25+1mg / kg) group, telmisartan+simvastatin+folic acid group according to the blood pressure value. (0.25+1+0.08mg / kg) group and a WKY control group, 20 rats in each group. Oral administration, once a day, weighed once a week, adjust the dose according to body weight, for 8 consecutive weeks.
[0091] Observation indicators:
[0092] (1) Observation of general state: daily observation of animal diet, survival and behavior, and comparison between groups.
[0093] (2) Blood pressure: Blood pressure was measured before administration, 4 weeks and 8 weeks after administration, and the effect of medication on blood pressure of hypertensive rats with hyperlipidemia was observed.
[0094] (...
Embodiment 3
[0110] Example 3: Target Organ Protection of Irbesartan + Pitavastatin + Folic Acid on Rats with Hypertension and Dyslipidemia
[0111] The compound model animals of 2K1C hypertension with dyslipidemia were prepared by the same method as in Example 1, and the hypertensive and dyslipidemia animals were selected for group administration, and the administration time was 16 weeks.
[0112] Observation indicators
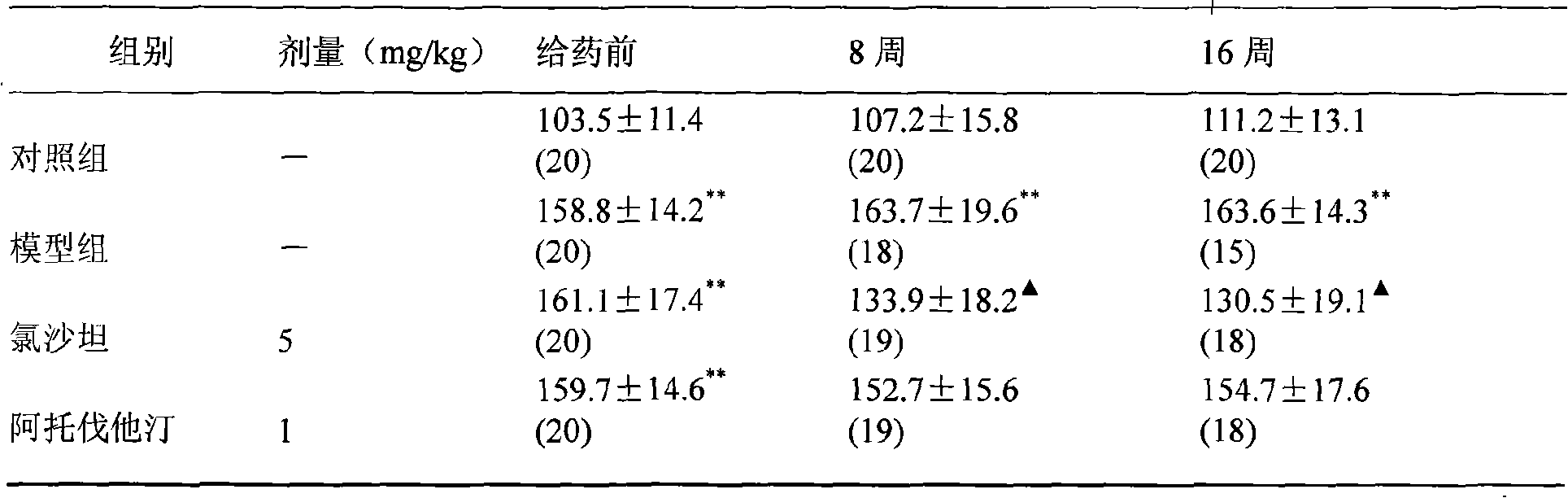
[0113] (1) Blood pressure: Blood pressure was measured before administration, 8 weeks and 16 weeks after administration, and the effect of medication on blood pressure in 2K1C hypertensive rats with hyperlipidemia was observed.
[0114] (2) Blood sugar and blood lipids: After the administration, blood was collected to measure plasma cholesterol (measured with a kit) and blood sugar levels by biochemical methods.
[0115] (3) Endothelial cells and antioxidant function: blood was collected as above, and serum NO, ET-1, SOD, MDA levels were determined by biochemical method...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com