Dextran fatty acid graft, preparation method and application
A fatty acid and dextran technology, applied in the field of preparation of anti-tumor pharmaceutical compositions, can solve the problems of unfavorable target cell uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
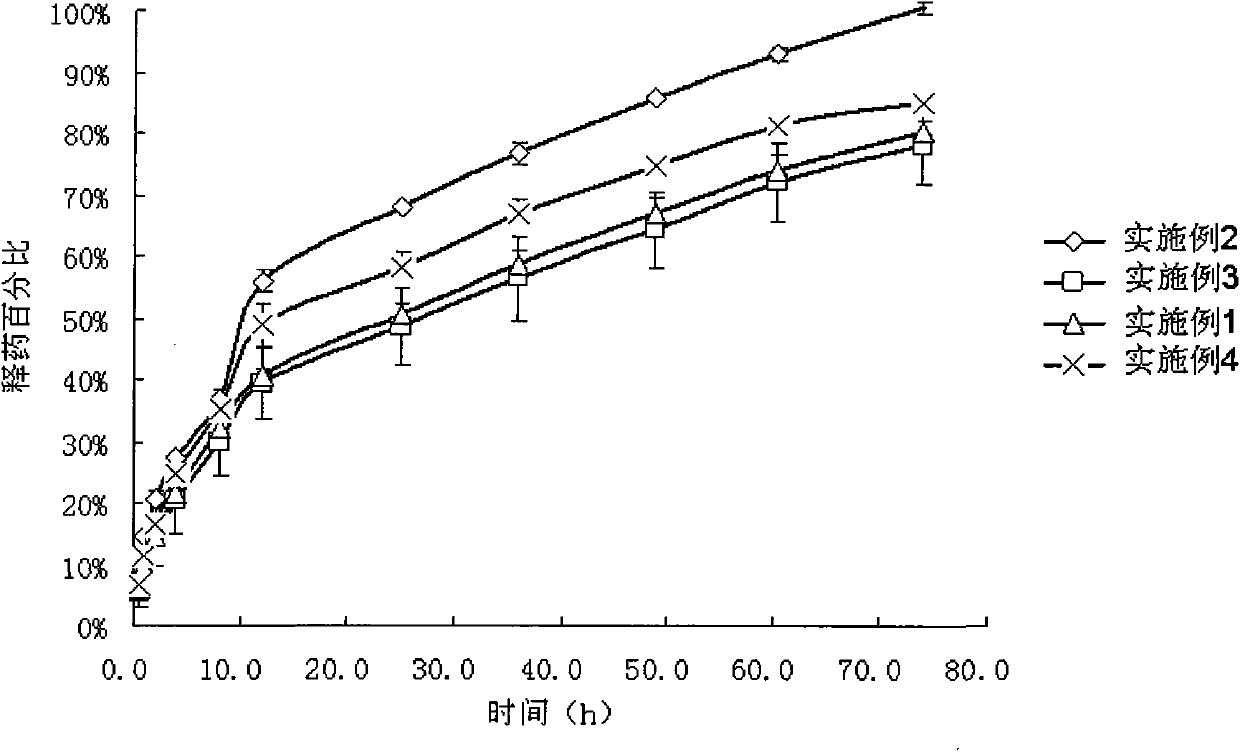
Examples
Embodiment 1
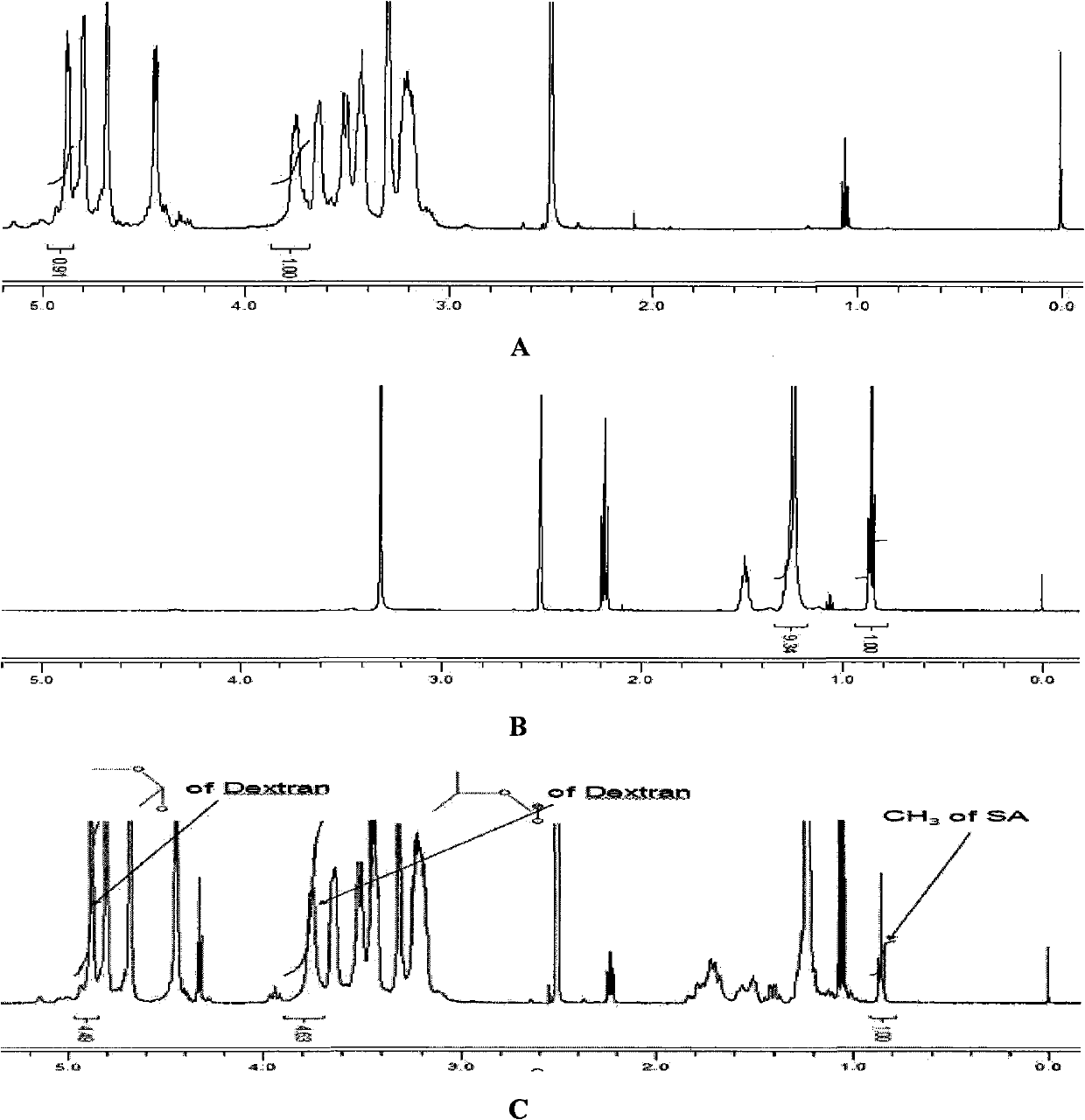
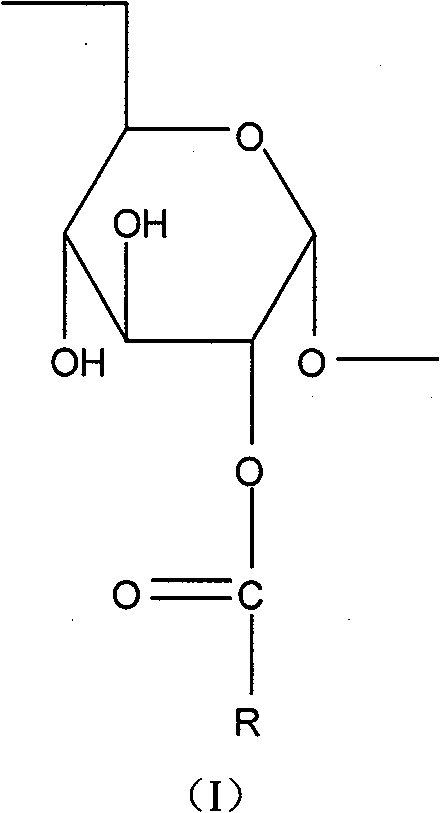
[0025] Accurately weigh 175.5mg of stearic acid, 381.8mg of DCC, 22.5mg of DMAP and 500mg of dextran (Mw=10000) into a 250ml dry round bottom flask, then add 45ml of dehydrated DMSO, heat and ultrasonically dissolve, add a small amount of anhydrous sodium sulfate. React at 60° C. for 8 h in a constant temperature heating magnetic stirrer, and protect with nitrogen. After the reaction, the reaction product was dialyzed for 48 hours with a dialysis bag (MWCO 3.5Kda, American Spectrum Laboratories Inc.), and the water was changed continuously to remove DMSO and water-soluble by-products. After dialysis, the reaction product was filtered with a 0.8 μm water membrane to remove insoluble substances such as DCC and then freeze-dried. The freeze-dried product was washed three times with ethanol to remove unreacted stearic acid. Finally, the reaction product is dissolved in deionized water and freeze-dried to obtain the dextran stearic acid graft
Embodiment 2
[0027] Accurately weigh 87.8mg of stearic acid, 191.5mg of DCC, 11.2mg of DMAP and 500mg of dextran (Mw=20000) into a 250ml dry round bottom flask, then add 45ml of dehydrated DMSO, heat and ultrasonically dissolve, add a small amount of anhydrous sodium sulfate. React at 60° C. for 8 h in a constant temperature heating magnetic stirrer, and protect with nitrogen. After the reaction, the reaction product was dialyzed for 48 hours with a dialysis bag (MWCO 3.5Kda, American Spectrum Laboratories Inc.), and the water was changed continuously to remove DMSO and water-soluble by-products. After dialysis, the reaction product was filtered with a 0.8 μm water membrane to remove insoluble substances such as DCC and then freeze-dried. The freeze-dried product was washed three times with ethanol to remove unreacted stearic acid. Finally, the reaction product is dissolved in deionized water and freeze-dried to obtain the dextran stearic acid graft
Embodiment 3
[0029]Accurately weigh 175.5mg of stearic acid, 381.8mg of DCC, 22.5mg of DMAP and 500mg of dextran (Mw=20000) into a 250ml dry round bottom flask, then add 45ml of dehydrated DMSO, heat and ultrasonically dissolve, add a small amount of anhydrous sodium sulfate. React at 60° C. for 8 h in a constant temperature heating magnetic stirrer, and protect with nitrogen. After the reaction, the reaction product was dialyzed for 48 hours with a dialysis bag (MWCO 3.5Kda, American Spectrum Laboratories Inc.), and the water was changed continuously to remove DMSO and water-soluble by-products. After dialysis, the reaction product was filtered with a 0.8 μm water membrane to remove insoluble substances such as DCC and then freeze-dried. The freeze-dried product was washed three times with ethanol to remove unreacted stearic acid. Finally, the reaction product is dissolved in deionized water and freeze-dried to obtain the dextran stearic acid graft
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com