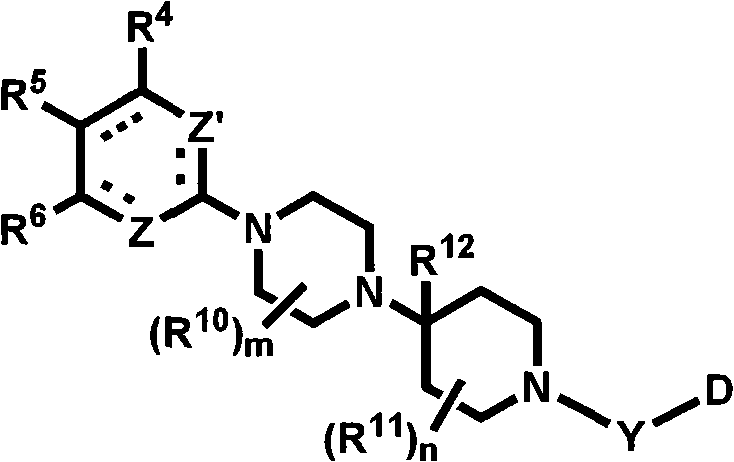
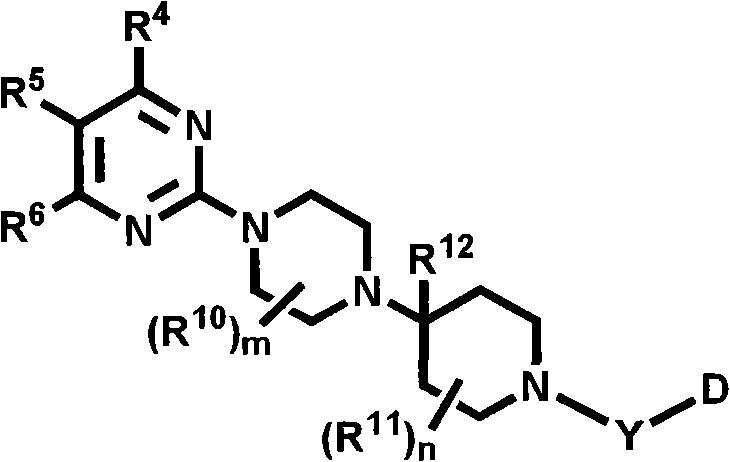
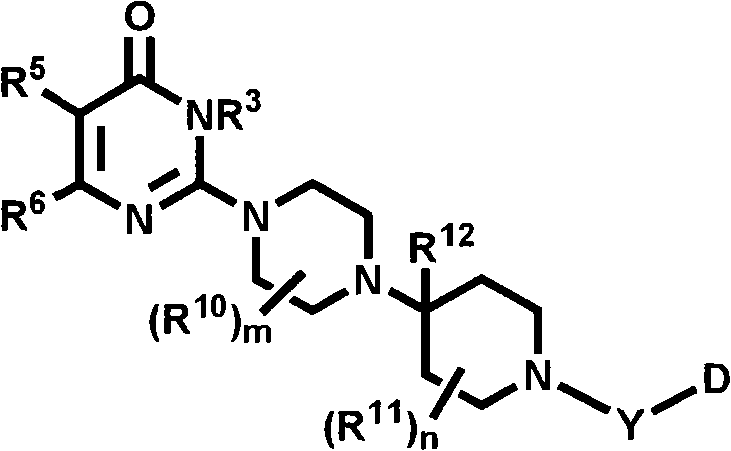
Heterocyclic compounds with cxcr3 antagonist activity
A compound, R30 technology, applied in the direction of organic active ingredients, sexual diseases, anti-inflammatory agents, etc., can solve problems such as unshown
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0440] Embodiment 1, step A, method A and method B
[0441]
[0442] A 500ml round bottom flask was charged with methyl 2-aminopyridine 3-carboxamide 1 (4.5g, 29.76mmol) and 1,2-dichloroethane (150ml). The resulting solution was cooled to -40°C while slowly adding triphosgene (7 g, 23.59 mmol). Triethylamine (4.4 g, 43.48 mmol) was then added dropwise via a syringe at this temperature. The reaction mixture was stirred at -40°C for two hours, then gradually warmed to room temperature and kept at this temperature overnight. The suspension was treated with water (100ml) and saturated sodium carbonate (100ml) and separated. The aqueous solution was extracted with dichloromethane. The combined organic layers were dried over sodium sulfate and dried on a rotary evaporator. The residue was dried under housevacuam to give a dark brown solid (4.1 g). This material was mixed with phosphorus oxychloride (50ml) in a 250ml flask. The resulting suspension was refluxed for 4 hours. ...
Embodiment 2
[0443] Embodiment 2, step B, method A and method B
[0444]
[0445] A round bottom flask was charged with crude material 2 (1.4 g, about 7 mmol), 2-S-ethylpiperazine (prepared according to Williams et al., J. Med. Chem 1996, 39, 1345; 80% active, 1.6 g, about 11 mmol), cesium carbonate (4.2 g, 12.9 mmol) and 1,4-dioxane (40 ml). The resulting suspension was stirred at room temperature for 5 days, diluted with dichloromethane (ca. 200 ml) and filtered through celite. The filtrate was washed once with water and then concentrated to an oil. The crude product was purified by silica gel chromatography using methanol / dichloromethane eluent (5% to 10% MeOH) to afford 0.7 g (9% based on compound 1) of the title compound.
Embodiment 3
[0446] Example 3. Step D, Method A
[0447]
[0448] Charge 4 (0.77g, 2.56mmol), 5 (1.4g, 7.03mmol), sodium triacetoxyborohydride (1.4g, 6.6mmol) and 1,2-dichloroethane into a 250ml round bottom flask (100ml). The resulting suspension was stirred at room temperature for 5 days and then quenched with 1.0 M sodium hydroxide solution. After separation, the aqueous solution was extracted with dichloromethane. The combined organic solutions were dried over sodium sulfate and concentrated under reduced pressure. Purification of the residue by flash chromatography on silica gel using 5% methanol in dichloromethane as eluent afforded the title compound (0.25 g, 21%) as a gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com