Improvement in synthesis of butylated hydroxyanisole from tertiary butyl hydroquinone
A technology of butylated hydroxyanisole and tert-butyl, which is applied in the directions of dehydration of hydroxyl-containing compounds to prepare ether, ether preparation, and ester reaction to prepare ether, etc., and can solve the problem that 3 isomers do not exceed 99%.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
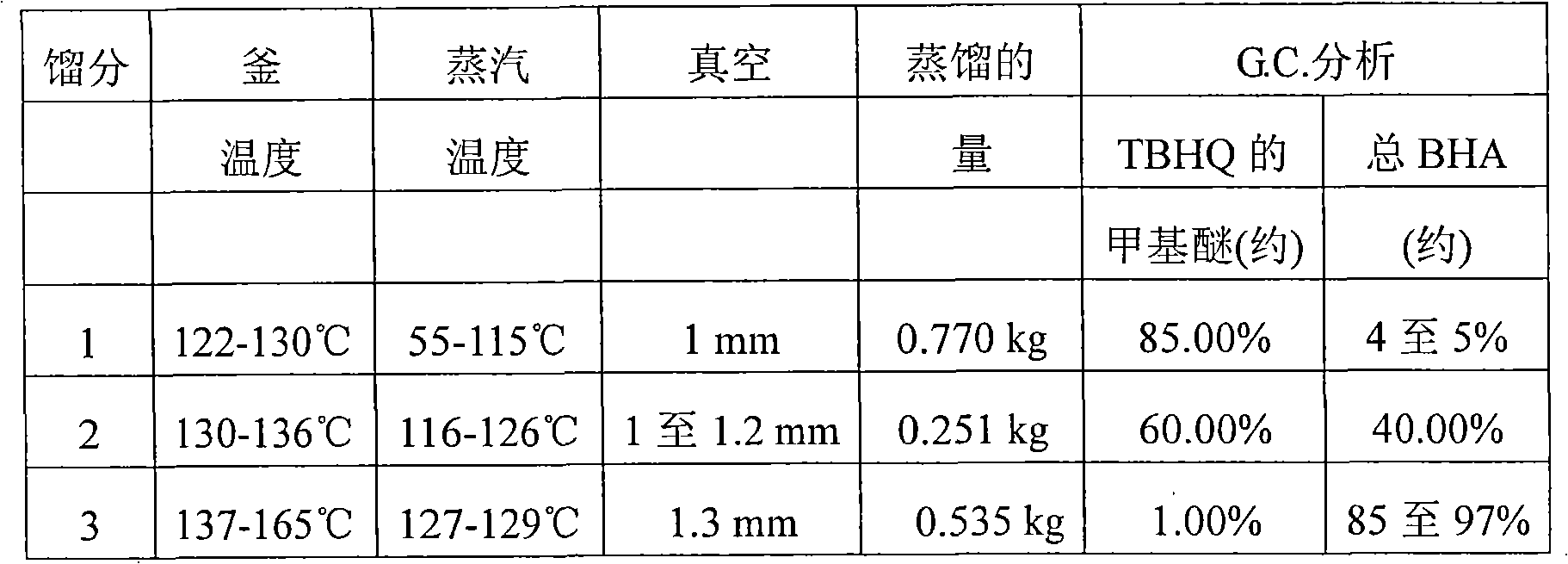
[0029]166 g of TBHQ suspended in 83 ml of water and 996 ml of hexane were placed in a 5 liter four-necked round bottom flask equipped with a stirrer, thermometer insert and dropping funnel, and stirred at 25-30° C. for 15-20 minutes. 159.32 g of dimethyl sulfate was added thereto and stirred for an additional 15 minutes. A solution of 55.6 g of sodium hydroxide in 111 ml of water was placed in a dropping funnel and added dropwise to the reaction mixture within 1 to 4.5 hours while maintaining the reaction temperature below 45-50°C. After the dropwise addition was complete, the reaction mixture was stirred at 25-30°C for a further 1 hour. The reaction was monitored by thin layer chromatography. The reaction mixture was then cooled to 20-25°C, adjusted to pH 3-4 with 50% sulfuric acid, and stirred at 25-30°C for a further 15-20 minutes. Then settle for 30 minutes.
[0030] The aqueous layer was then separated and discarded. The hexane layer was washed with 250 ml of water, i...
Embodiment 2
[0032] Scale-up test
[0033] Experiment 1 was repeated on a batch scale using 1000 kg or more of TBHQ per batch, with results obtained by varying the relative stoichiometric ratios of TBHQ, DMS, and NaOH to include the following ratios, respectively: (1:1.1:1.33) , (1:1.1:1.46), (1:1.26:1.38), (1:1.1.4:1.46), (1:1.26:1.4), (1:1.1:1.2) and (1:1.03:1.2) . In these ratios (1:1.26:1.38), (1:1.1.4:1.46) are considered close to the ideal situation, the conversion of TBHQ is almost 100% or very close to 100%, and a high yield of BHA is obtained, of which 3 - The purity of the isomers was 99.5% or higher and (1:1.26:1.38) was found to be superior in practice. Of course, any other stoichiometric ratio of TBHQ, DMS and NaOH in which DMS is slightly in excess of TBHQ and NaOH is slightly in excess of DMS can be used, although the above ratios have been identified as preferred ratios, any other stoichiometric ratio It should also be within the scope of the present invention.
Embodiment 3
[0035] BHA in Different Physical Forms
[0036] Large-scale production according to the stoichiometric ratio described in Example 1 resulted in a slurry in which BHA was isolated in crystalline form by cooling the final hexane layer.
[0037] The crystallized solid was isolated by passing the slurry through a Nutsche filter with stirring to separate the crystals.
[0038] The crystalline form gives rise to two other physical forms, a flake form and a compressed form.
[0039] The flake form is formed when the crystals are melted by passing steam through a jacket, and the molten BHA is passed through a flaker machine with cold water circulation. After cooling, the BHA solidifies into flake form during this treatment.
[0040] The crystalline BHA isolated by the Nutsche filter is formed into a compressed form, such as tablets of various sizes, when passed through a tablet press well known in the art, and tablets of various sizes are formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com