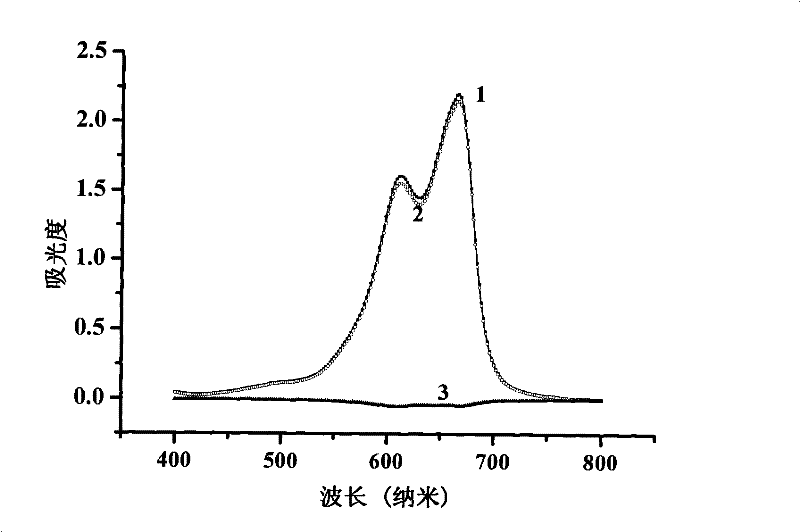
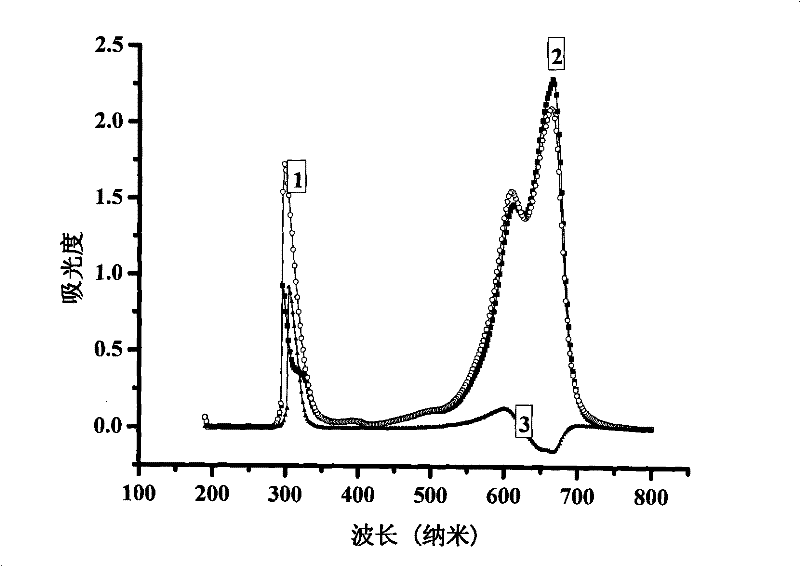
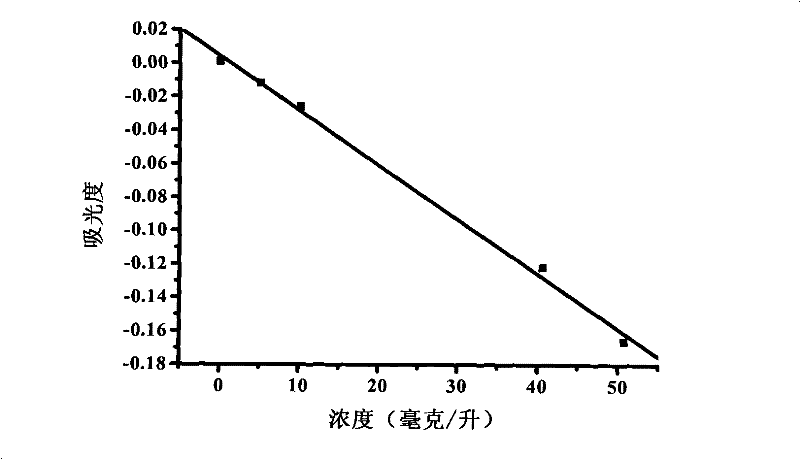
Method for measuring contents of anionic surface active substances by methylene blue spectrometry
A surface active material, methylene blue technology, applied in the direction of color/spectral property measurement, etc., to achieve the effect of good reproducibility, stable absorbance and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Capacity bottle 25ml, 50ml
[0042] Implip 10ml
[0043] The balance of the balance of 220g, the sensor 0.1mg
[0044] Acidal meter accuracy ± 0.01
[0045] UV-Visible Lighting Optimization of Optical accuracy ± 0.002ABS
[0046] 2. Reagent
[0047] Armor blue test agent analysis pure
[0048] Sodium sodium sulfate analysis pure
[0049] Sodium acetate analysis pure
[0050] Pure ice acetic acid analysis
[0051] Ethanol analysis pure
[0052] 3. Experimental steps
[0053] (1) Dissan sample: Accurately referred to as a magnesium carbonate that does not contain sodium sodium sodium sulfate, and 2.5649g each of the alkaline -type magnesium carbonate sample containing sodium sodium sulfate, the quality error of the two samples is controlled at 0.2 mg of 0.2 mgInside.With a 35 % concentration of nitric acid solution solution, the solution processes are performed in a closed container, and the solution with a concentration of alkaline magnesium carbonate is 0.129mol / L.
[0054] (...
Embodiment 2
[0076] Same as Examples
[0077] 2. Reagent
[0078] Sodium sodium sodium sulfate is replaced with potassium hydrogenate (benchmarks) of phthalate, and other examples are the same as the embodiment 1.
[0079] 3. Experimental steps
[0080] (1) Dissan sample: Accurately referred to as a magnesium carbonate that does not contain potassium hydrogen acid hydrogenate, and 2.5649g of alkali carbonate samples containing hydrogen carbonate containing pyrodiamate, the quality error of the two samples is controlled within 0.2 mgEssenceUse a 35 % nitrate solution for soluble samples, and the solution process is performed in a closed container, which is prepared into a solution with a concentration of 0.129mol / L.
[0081] (2) Preparation of sodium acetate-sodium acetate buffer: Same as Example 1.
[0082] (3) Preparation of Armo -based blue solution: Same as Example 1.
[0083] (4) Preparation of potassium hydrogen acid hydrogenate: 0.1021g of potassium hydrogen acid hydrogenate, dissolved w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com