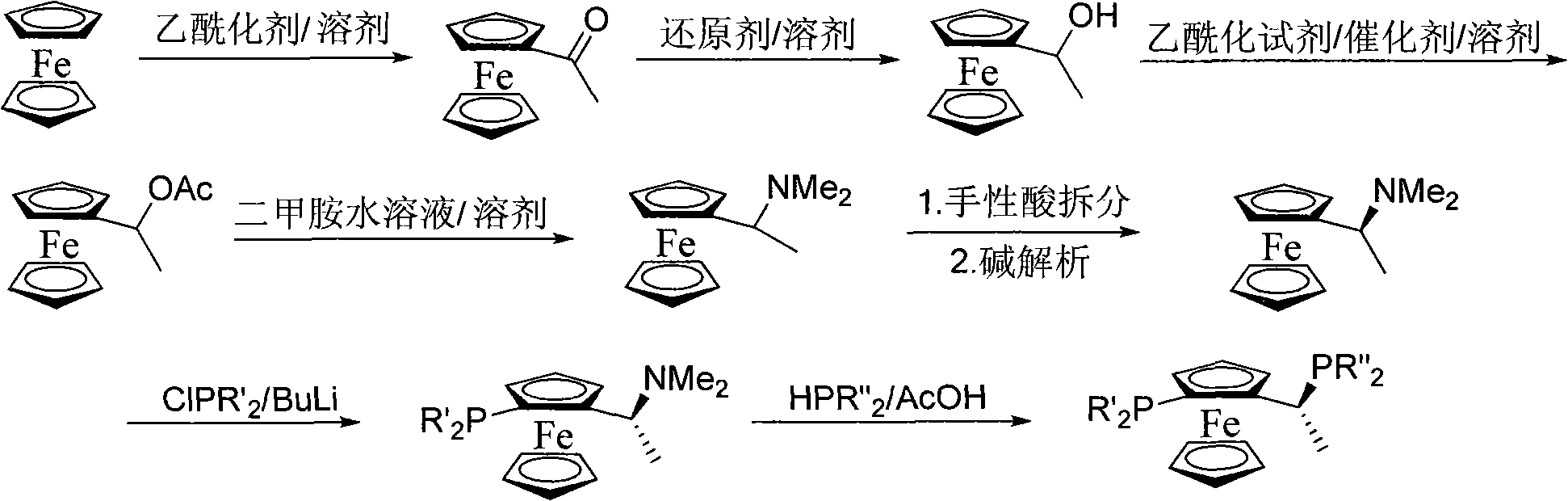
Method for synthesizing chiral ferrocene diphosphine ligand
A technology of chiral ferrocene and synthesis method, which is applied in the field of synthesis of chiral ferrocene bisphosphine ligands, can solve problems such as inconvenient operation, and achieve the effects of simplified operation, high quality, and meeting the requirements of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The synthesis method of bisphosphine ligand is achieved through the following steps:
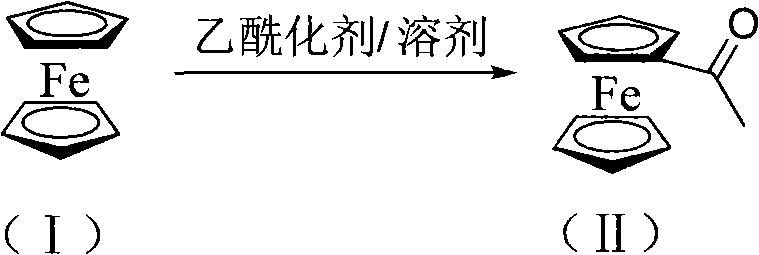
[0060] A) Synthesis of acetylferrocene(II)
[0061] Add 18.6g ferrocene (I), 14g aluminum trichloride, and 60ml petroleum ether into a 250ml three-necked flask with mechanical stirring, reflux and dropping funnel, and slowly add 11ml of acetyl chloride in about 30 minutes. Finish dripping. After reacting at 50°C for 4 hours, the reaction solution was poured into water, the organic layer was separated, the aqueous layer was extracted with the organic solvent dichloromethane, the organic layers were combined, dried, and concentrated to obtain about 21 g of pure acetylferrocene (II). The rate is 92%. Melting point 83-85°C.
[0062] 1 H NMR(CDCl 3 ): δ4.77 (s, 2H), 4.50 (s, 2H), 4.21 (s, 4H), 2.40 (s, 3H); 13 C NMR (CDCl): δ 201.9, 79.0, 72.2, 69.7, 69.4, 27.2.
[0063] 2) Preparation of ferrocene ethanol (III)
[0064] Add 22.8g of acetylferrocene (II), 38ml of methanol, and 5.4g of potassiu...
Embodiment 2
[0086] The synthesis method of bisphosphine ligand is achieved through the following steps:
[0087] A) Synthesis of acetylferrocene (II)
[0088] 18.6g ferrocene (I), 12g alumina, 60ml dichloromethane were added to a 250ml three-necked flask connected with mechanical stirring, reflux and dropping funnel, and 21ml acetyl chloride was added dropwise within 30 minutes. After reacting at 60°C for 5 hours, the reaction solution was poured into 100ml of water, the organic layer was separated, the aqueous layer was extracted with organic solvent dichloromethane, the organic layers were combined, dried and concentrated to obtain about 21.9g pure acetylferrocene(II) , The yield is 96%. Melting point 83-85°C.
[0089] 1 H NMR(CDCl 3 ): δ4.77 (s, 2H), 4.50 (s, 2H), 4.21 (s, 4H), 2.40 (s, 3H); 13 C NMR (CDCl): δ 201.9, 79.0, 72.2, 69.7, 69.4, 27.2.
[0090] 2) Preparation of ferrocene ethanol (III)
[0091] Add 21.9g of acetylferrocene (II), 38ml of methanol, 2.3g of sodium to a 250ml single...
Embodiment 3
[0113] The synthesis method of bisphosphine ligand is achieved through the following steps:
[0114] A) Synthesis of acetylferrocene (II)
[0115] 18.6g ferrocene (I), 13.6g zinc chloride, and 60ml toluene were added to a 250ml three-necked flask with mechanical stirring, reflux and dropping funnel, and 21ml acetyl chloride was slowly added dropwise within 30 minutes. After reacting at 60°C for 5 hours, the reaction solution was poured into 100 ml of water, the organic layer was separated, the aqueous layer was extracted with the organic solvent dichloromethane, the organic layers were combined, dried, and concentrated to obtain about 22 g of pure acetylferrocene (II). The yield was 96.5%. Melting point 83-85°C.
[0116] 1 H NMR(CDCl 3 ): δ4.77 (s, 2H), 4.50 (s, 2H), 4.21 (s, 4H), 2.40 (s, 3H); 13 C NMR (CDCl): δ 201.9, 79.0, 72.2, 69.7, 69.4, 27.2.
[0117] 2) Preparation of ferrocene ethanol (III)
[0118] Add 2.28 g of acetylferrocene (II), 4 ml of tetrahydrofuran, and 10 ml of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com