Cefmenoxime hydrochloride composition powder injection and manufacturing method thereof
A technology of cefmenoxime hydrochloride and its composition, which is applied in the field of cefmenoxime hydrochloride composition powder injection and its preparation, can solve problems such as accelerated drug decomposition and molecular rearrangement of cefmenoxime, and achieve quality improvement, rapid dissolution and stability sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
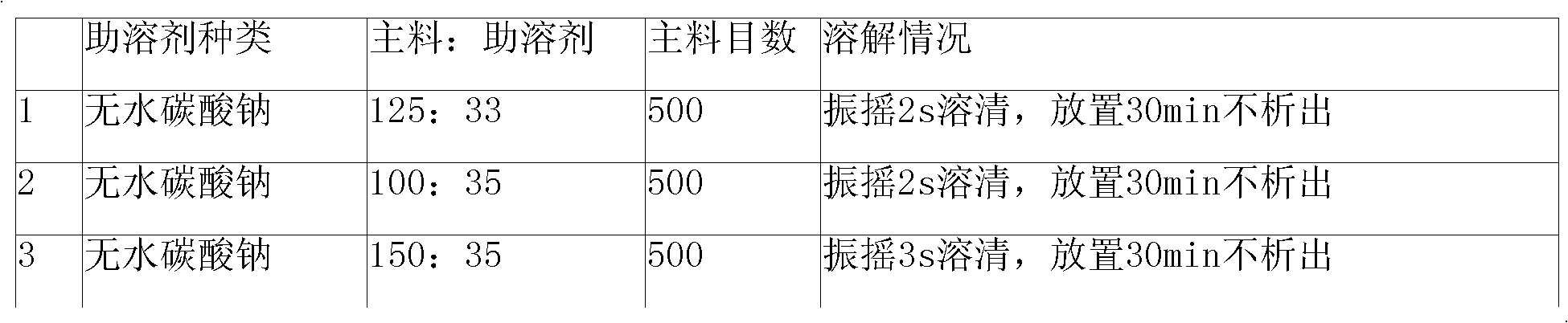
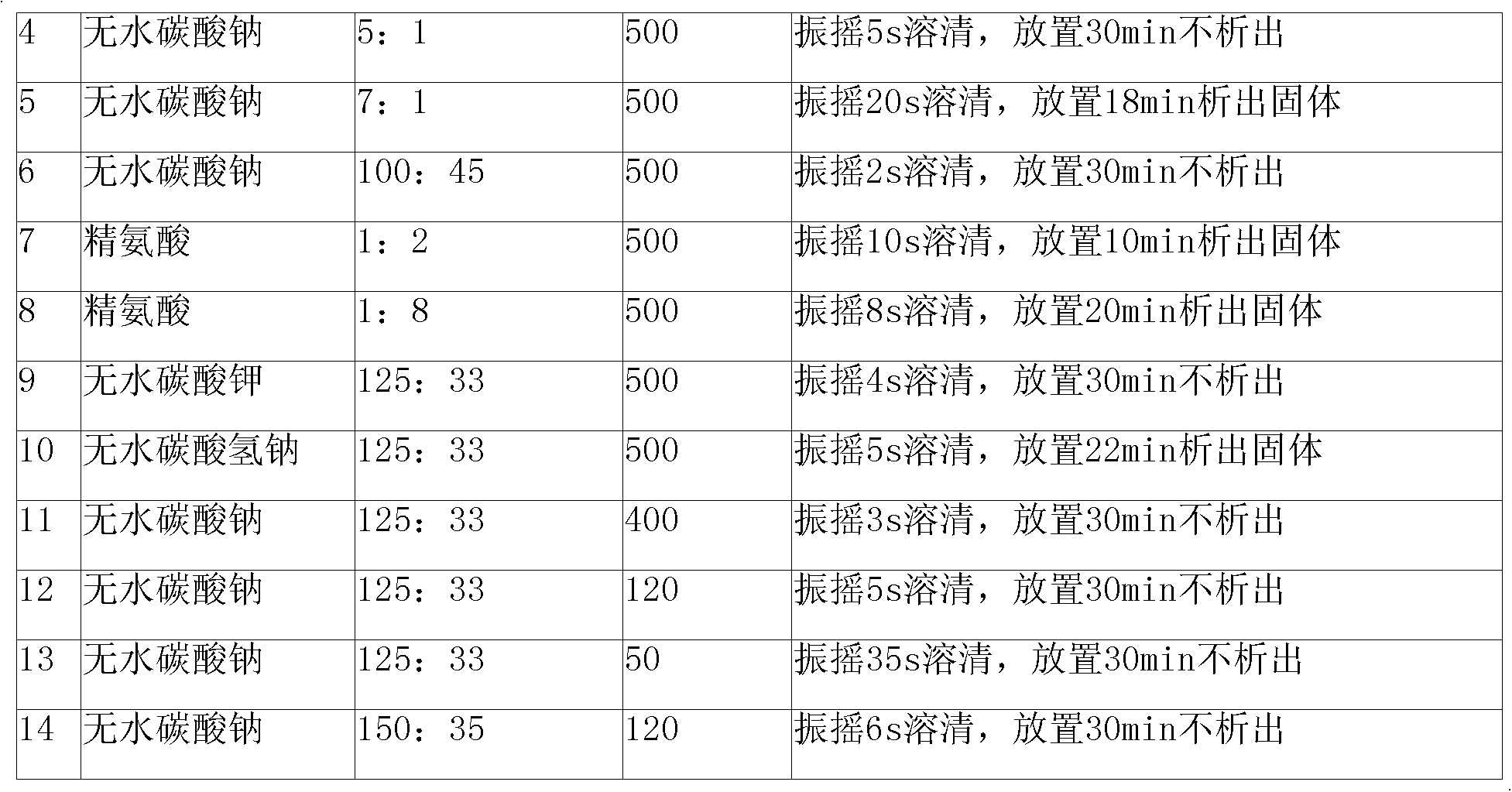
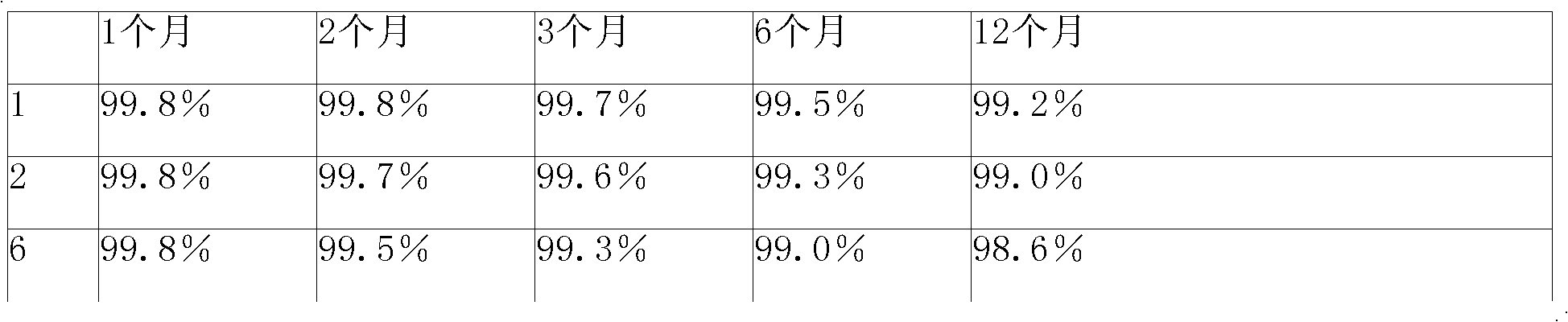
[0060] 250g of cefmenoxime hydrochloride and 66g of anhydrous sodium carbonate were respectively passed through a 200 mesh sieve, then mixed uniformly and pulverized using a ball mill filled with nitrogen, nitrogen protection during the pulverization process, pulverization until the particle size of cefmenoxime hydrochloride was 500 mesh, after pulverization, use The three-dimensional mixer is mixed, and the speed of the mixer is 60 rpm, and the mixture is mixed for 25 minutes. After the mixing is completed, it is divided into 1000 bottles of sterilized vials and stoppered.
[0061] Or recrystallize cefmenoxime hydrochloride before powder injection preparation: dissolve cefmenoxime hydrochloride with an HPLC content of 99.5% in 10 times aseptically treated acetone at 40°C, adjust the stirring speed to 80 rpm, and slowly drop 1 times sterilized water for injection, the dropping time is 3.2min, after dropping, adjust the stirring speed to 47 rpm, cool down to -5°C, let it stand o...
Embodiment 2
[0063] 500 g of cefmenoxime hydrochloride and 132 g of anhydrous sodium carbonate were passed through a 200 mesh sieve respectively, then mixed uniformly and pulverized using a ball mill filled with nitrogen, nitrogen protection during the pulverization process, pulverization until the particle size of cefmenoxime hydrochloride was 500 mesh, after pulverization, use The three-dimensional mixer is used for mixing, the speed of the mixer is 50 rpm, and the mixture is mixed for 30 minutes. After the mixing is completed, it is divided into 1000 bottles of sterilized vials and corked.
[0064] Or recrystallize cefmenoxime hydrochloride before powder injection preparation: dissolve cefmenoxime hydrochloride with an HPLC content of 99.5% in 10 times aseptically treated acetone at 40°C, adjust the stirring speed to 80 rpm, and slowly drop 1 times sterilized water for injection, the dropping time is 4.5min, after dropping, adjust the stirring speed to 47 rpm, lower the temperature to 0°...
Embodiment 3
[0066] 1000g of cefmenoxime hydrochloride and 264g of anhydrous sodium carbonate were passed through a 200 mesh sieve respectively, then mixed uniformly and pulverized using a ball mill filled with nitrogen, nitrogen protection during the pulverization process, pulverization until the particle size of cefmenoxime hydrochloride was 500 mesh, after pulverization, use The three-dimensional mixer is used for mixing, the speed of the mixer is 50 rpm, and the mixture is mixed for 25 minutes. After the mixing is completed, it is packed into 1000 sterilized vials and corked.
[0067] Or recrystallize cefmenoxime hydrochloride before powder injection preparation: dissolve cefmenoxime hydrochloride with an HPLC content of 99.5% in 10 times aseptically treated acetone at 40°C, adjust the stirring speed to 80 rpm, and slowly drop 1 times sterilized water for injection, the dropping time is 6.3min, after dropping, adjust the stirring speed to 47 rpm, cool down to 0°C, let it stand overnight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com