Chemical methods and apparatus
A microfluidic device and compound technology, applied in chemical instruments and methods, organic chemistry, peptide preparation methods, etc., can solve the problems of lack of rapid and general methods for peptide and biomolecular labeling, hindering peptides and biomolecules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: [ 18 Preparation of F]-4-(2-fluoroethyl)-triazol-1-yl-[KGFGK] using a copper ring reactor
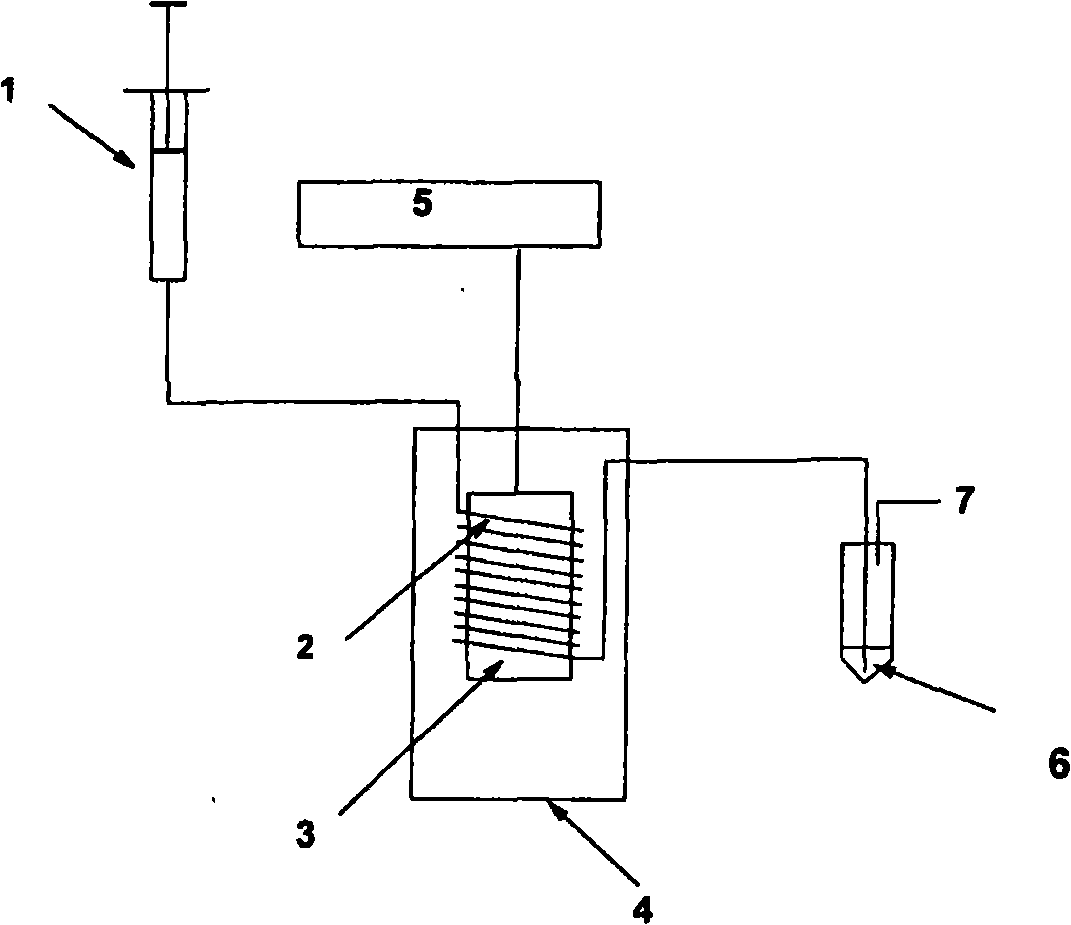
[0086] This example refers to figure 1 to describe. The heated copper tube is 1.0 m long, with an inner diameter of 0.56 mm and a tube volume of 246 μl).
[0087]
[0088] Solution of model peptide 1 (2.4mg, 4.08μmol), sodium phosphate buffer (0.2ml, pH 6.0, 250mM), DMF (0.05ml) and [18F]2-fluoroethyl azide (0.6mCi, 23MBq) Mix in acetonitrile (0.2ml). The labeling mixture was filled into a Hamilton-sealed glass syringe (1 ) and then passed through a copper ring (2) at 80° C. at a flow rate of 0.2 ml / min under the action of a pump. The electric heating cylinder (3) is heated to 200°C by a heating module (4) with a temperature control unit (5). The reaction mixture is collected in a vial (6) equipped with an outlet (7). Analysis of the reaction mixture by HPLC showed formation of 2 in 85% radiochemical yield after about 3-4 minutes. Reinjection of the labeling...
Embodiment 2
[0090] Embodiment 2: The preparation of compound 20 adopts copper ring reactor
[0091] Compound 20 was prepared from Compound 19 with reference to Comparative Example 12.
[0092] Compound 19 (2.9 mg, 2.04 μmol) was dissolved in sodium phosphate buffer (100 μl, pH 6.0, 100 mM) added with dimethylformamide (25 μl). After addition of compound 11 (518 μCi / 19 MBq) in acetonitrile (100 μl), the mixture was pumped at 0.1 ml / min into a preheated copper ring reactor at 80°C. Subsequently, the system was rinsed with water (0.5 ml). HPLC analysis of the first and second fractions revealed labeling efficiencies of 9% and 34%, respectively. The total radioactivity recovered from the system was 53%.
Embodiment 3
[0093] Embodiment 3: [ 18 Preparation of F]-4-(2-fluoroethyl)-triazol-1-yl-[KGFGK] using copper loop reactor
[0094] A solution of model peptide 1 (2.4mg, 4.08μmol), sodium phosphate buffer (0.2ml, pH 6.0, 250mM), DMF (0.05ml) with [ 18 F] 2-Fluoroethyl azide (0.9 mCi, 34 MBq) was mixed in acetonitrile (0.2 ml). The mixture was pumped into the heating copper ring as in Example 1, but with a flow rate of 0.1 ml / min. The flow-through time of the mixture was 3 minutes and the total reaction time was 10 minutes. Labeled peptide 2 was collected in 77% (decay corrected) recovery. Radiochemical purity was >99%. The copper ring reactor was washed with water (1ml), water / TFA 1 / 1 (2ml), water (2ml), acetonitrile (3ml) and dried with nitrogen flow (1ml, 50ml / min). Using the same active [ 18 F] 2-Fluoroethyl azide Repeat experiment. Isolate 2 had a radiochemical yield of 71% (decay corrected) and a radioactive purity of 98%.
[0095] Preparation of control compounds
[009...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com