Method for modifying high-rate lithium-rich anode material
A technology of lithium-rich cathode materials and cathode materials, which is applied in electrode manufacturing, battery electrodes, electrical components, etc., can solve problems such as difficult industrialized large-scale production, obstacles to large-scale applications, and poor rate performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. First, according to the molecular formula Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 The ratio of Ni and Mn in the preparation of NiSO 4 and MnSO 4 Mixed solution, the cation concentration is 2mol / L;
[0023] 2. Use a peristaltic pump to drop the mixed solution and LiOH solution into the reaction kettle, and control the pH value at about 11, and heat it in a water bath to 60°C. After the reaction is completed, filter, wash, and dry in a vacuum oven at 120°C for 12 hours to obtain the precursor M(OH) 2 (M=Mn, Ni):
[0024] 3. Combine the precursor with LiOH·H 2 O according to the molecular formula Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 After mixing evenly, keep the temperature at 450°C for 6h in the air atmosphere, continue to heat up to 950°C for 10h, and cool to room temperature with the furnace to obtain the lithium-ion battery cathode material Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 ;
[0025] 4. Disperse the lithium-rich cathode material obtained above in 0.194g / L MnSO 4 The solut...
Embodiment 2
[0030] 1-3 steps are the same as embodiment 1;
[0031] 4. Disperse the obtained lithium-rich cathode material in 0.777g / L MnSO 4 The solution was sonicated for 1h, and vigorously stirred for 2h, then 0.487g / L Na 2 CO 3 The solution was dripped into the strongly stirred MnSO4 through a peristaltic pump 4 solution, after dripping, filter the solution and dry it at 120°C;
[0032] 5. Then sinter at 300°C for 6 hours to obtain surface-modified Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 .
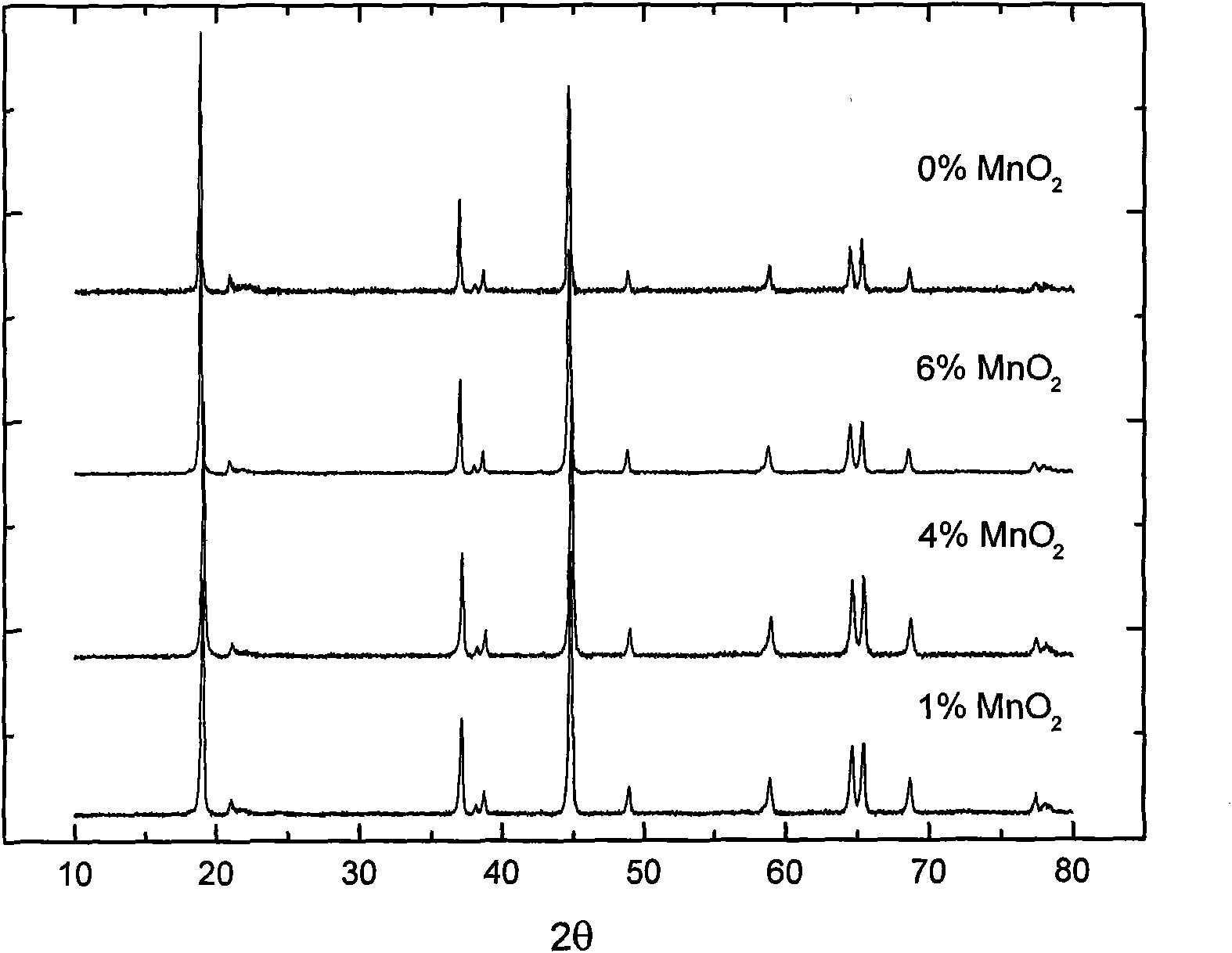
[0033] X-ray diffraction (XRD) analysis showed that the main phase of the product was Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 (See figure 1 ), its structure was not damaged after coating.
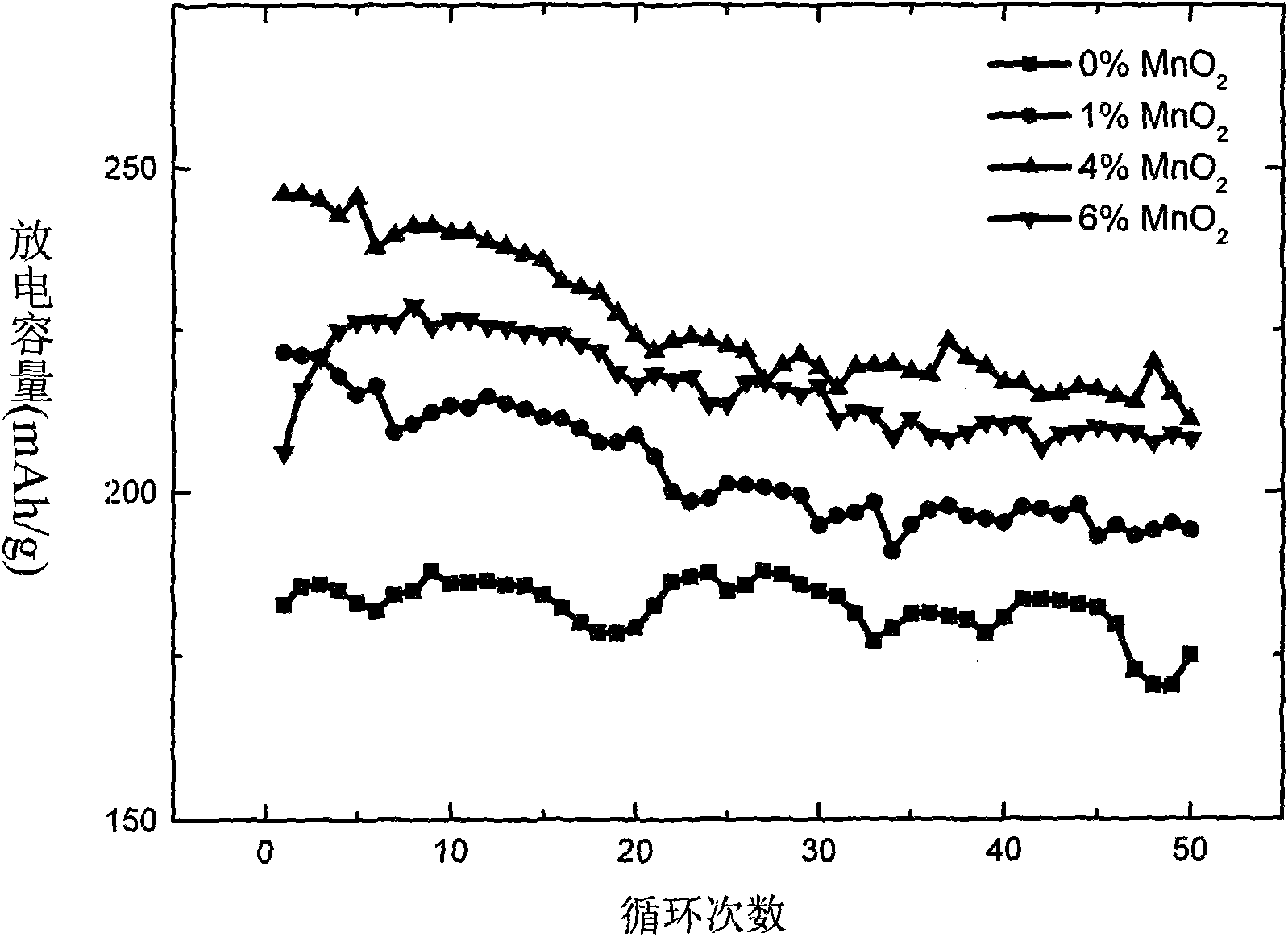
[0034] Electrochemical tests show that the first discharge capacity is 192mAh / g at 0.5C (see figure 2 ), the capacity of 190mAh / g is still maintained after 50 cycles, and the capacity retention rate is as high as 98.9%. attenuation.
Embodiment 3
[0036] 1-3 steps are the same as embodiment 1;
[0037] 4. Disperse the obtained lithium-rich cathode material in 0.777g / L MnSO 4 The solution was sonicated for 1h, and vigorously stirred for 2h, then 0.487g / L Na 2 CO 3 The solution was dripped into the strongly stirred MnSO4 through a peristaltic pump 4 solution, after dripping, filter the solution and dry it at 120°C;
[0038] 5. Then sinter at 500°C for 6h to obtain surface-modified Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 .
[0039] X-ray diffraction (XRD) analysis showed that the main phase of the product was Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 (See figure 1 ), its structure was not damaged after coating. However, MnCO 3 The product decomposed at 500°C is not MnO 2 , but Mn 5 o 8 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com