Novel tricyclic spiropiperidine compounds, their synthesis and their uses as modulators of chemokine receptor activity
A compound, piperidine technology, applied in the field of preparation of the compound, can solve problems such as dyspnea, insufficient blood oxygen supply, etc., and achieve a good effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
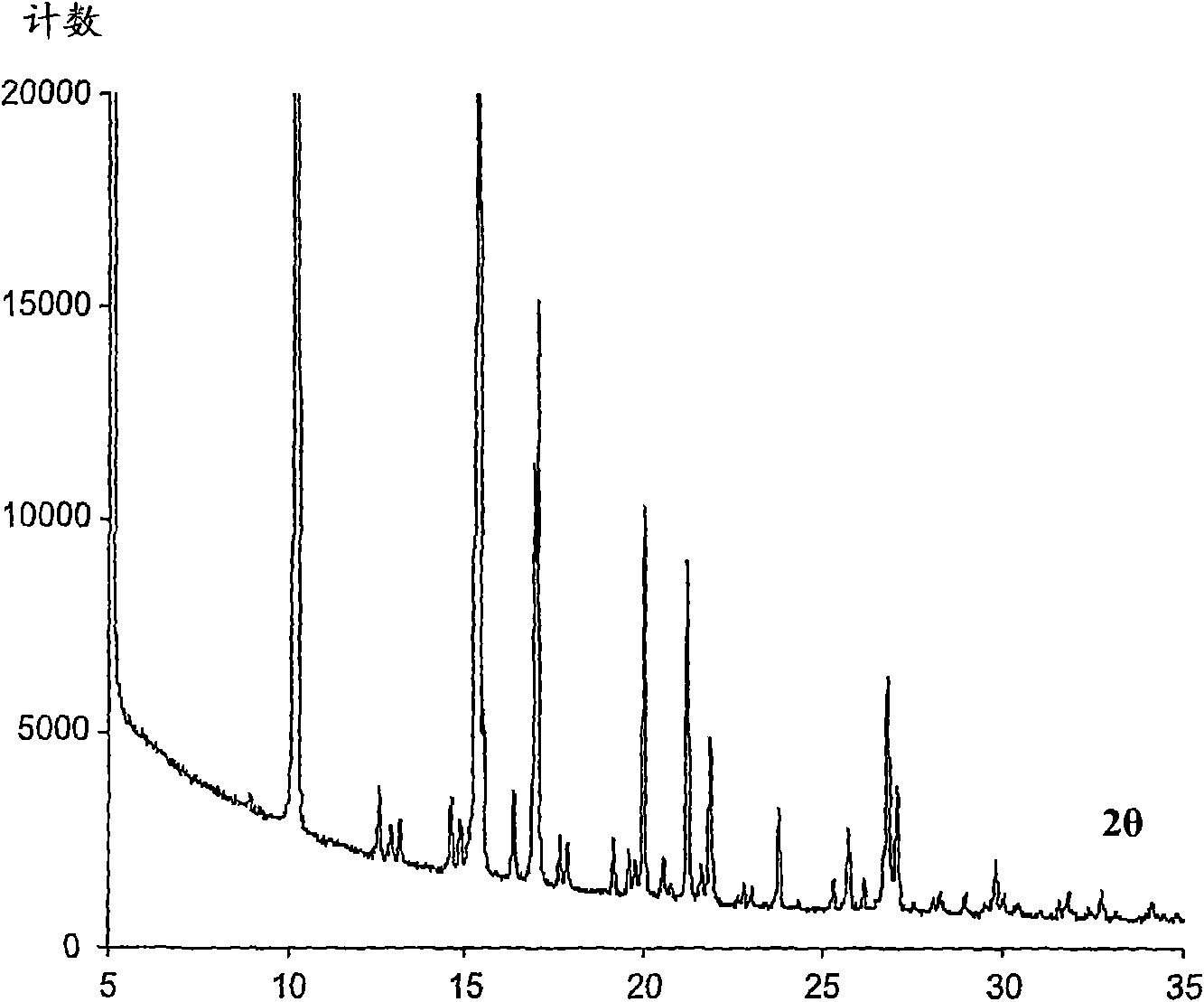
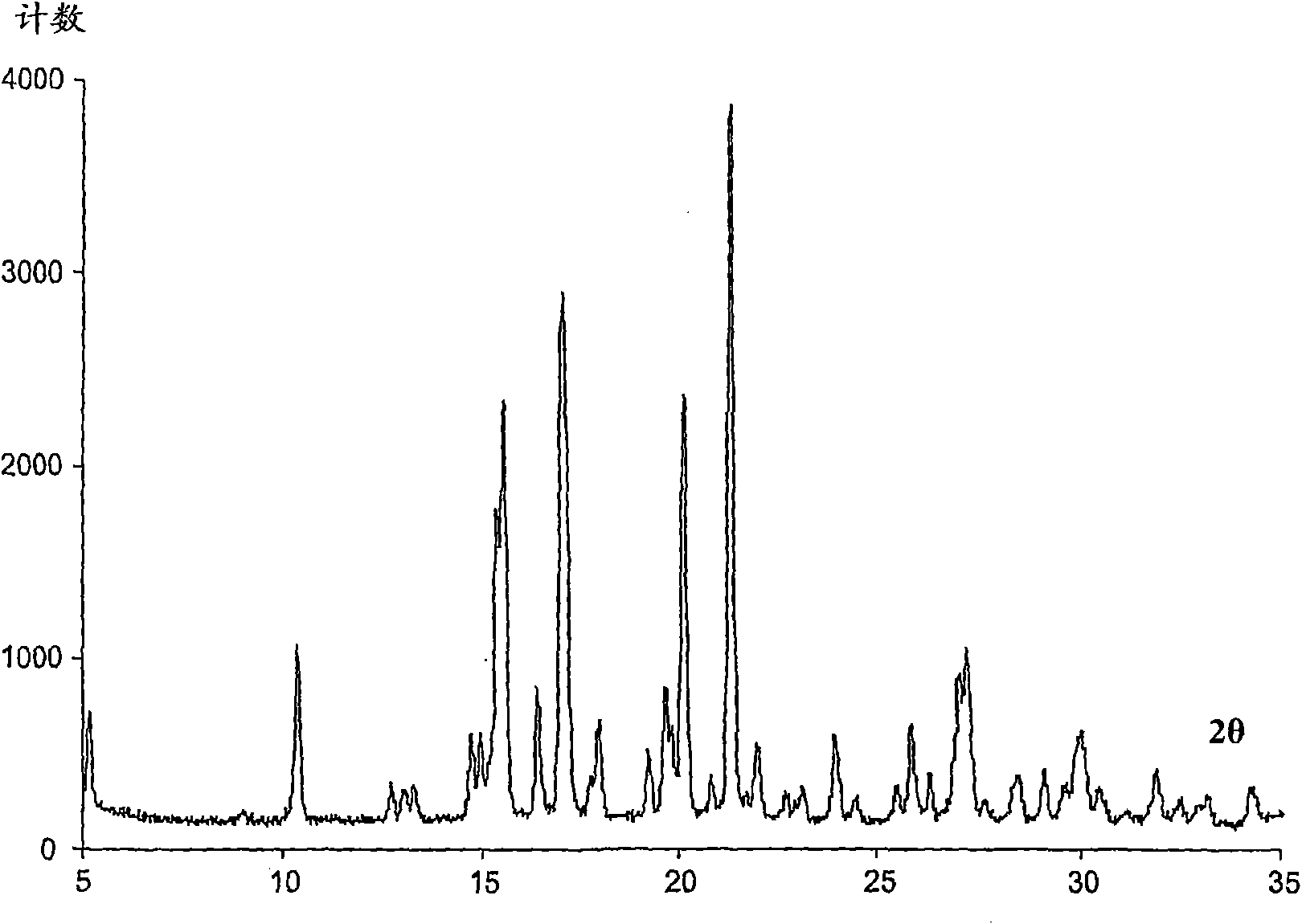
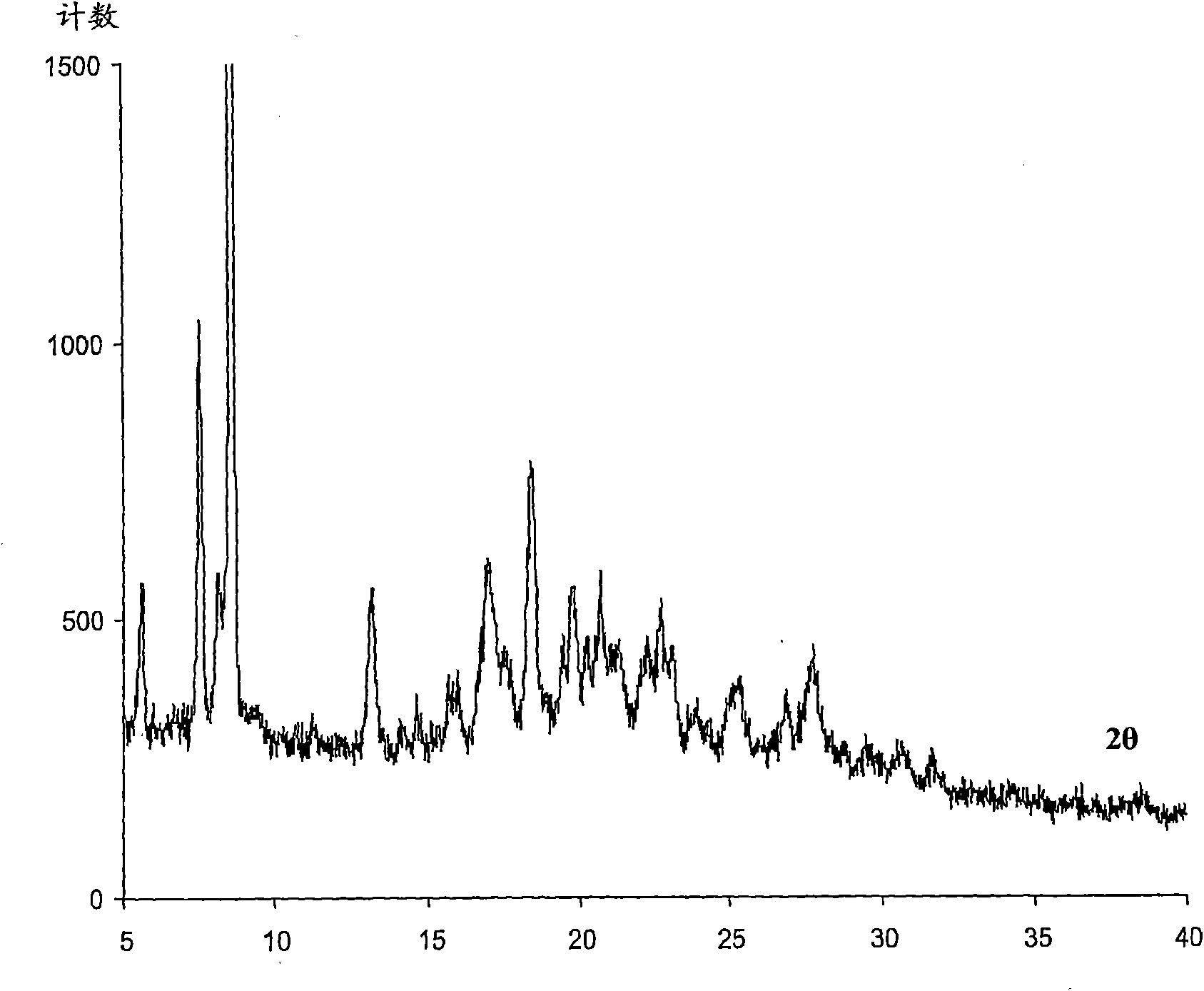
Image
Examples
Embodiment 1
[0522] (4-(acetylamino)-2-chloro-5-{[(2S)-3-(5-chloro-1’H,3H-spiro[1-benzofuran-2,4’-piper Pyridine]-1'-yl)-2-hydroxypropyl]oxy}phenoxy)acetic acid
[0523] Step 1: {5-Chloro-4-[(4-methoxybenzyl)oxy]-2-[((2S)-oxiran-2-yl)methoxy]phenyl}carbamate tert-butyl ester
[0524] To a solution of tert-butyl {5-chloro-2-hydroxy-4-[(4-methoxybenzyl)oxy]phenyl}carbamate (2.9 g) in NMP (20 ml) was added cesium carbonate ( 2.6 g) and 3-nitrobenzenesulfonic acid (2S)-oxiran-2-ylmethyl ester (1 equivalent). The reaction mixture was stirred at room temperature for 18 h, then partitioned between diethyl ether and water. The organic phase was dried over sodium sulfate, and then the solvent was removed to obtain 3.3 g (99%) by APCI-MS (m / z 435 (M + )) identified subtitle compound as a flowable oil.
[0525] Step 2: 5-chloro-2-{[(2S)-3-(5-chloro-1'H,3H-spiro[1-benzofuran-2,4'-piperidin]-1'-yl) -2-Hydroxypropyl]oxy}-4-hydroxyphenylcarbamate tert-butyl ester
[0526] To {5-chloro-4-[(4-met...
Embodiment 2
[0534] (4-(acetylamino)-3-{[(2S)-3-(5-chloro-1’H,3H-spiro[1-benzofuran-2,4’-piperidine]-1’- base)-2-hydroxypropyl]oxy}phenoxy)acetic acid
[0535] Method A
[0536] Step 1: (4-Acetylamino-5-{[(2S)-3-(5-chloro-1'H,3H-spiro[1-benzofuran-2,4'-piperidine]-1' -yl)-2-hydroxypropyl]oxy}phenoxy)methyl acetate
[0537] To N-[2-[(2S)-3-(5-chlorospiro[benzofuran-2(3H),4'-piperidin]-1'-yl)-2-hydroxypropoxy]-4 To a solution of -hydroxyphenyl]acetamide (45 mg) in DMF (1 ml) was added cesium carbonate (49 mg) and methyl bromoacetate (15 mg). The reaction mixture was stirred at room temperature for 6 h after which time the mixture was filtered and then purified by reverse phase HPLC (water: acetonitrile with 1% TFA) to afford 21 mg (33%) of the subtitle compound as a white solid.
[0538] 1 H-NMR (d6-DMSO) δ9.57-9.48 (m, H), 8.94 (d, J=8.3, NH), 7.67-7.64 (m, H), 7.31-7.29 (m, H), 7.17- 7.15 (m, H), 6.82-6.78 (m, H), 6.69-6.68 (m, H), 6.51-6.48 (m, H), 6.03 (b, OH), 4.78 (s, 3H), 4...
Embodiment 3
[0548] (4-(acetylamino)-2-chloro-5-{[(2S)-3-(5-fluoro-1’H,3H-spiro[1-benzofuran-2,4’-piper Pyridine]-1'-yl)-2-hydroxypropyl]oxy}phenoxy)acetic acid hydrochloride
[0549] Step 1: N-(5-chloro-2-{[(2S)-3-(5-fluoro-1'H,3H-spiro[1-benzofuran-2,4'-piperidine]-1' -yl)-2-hydroxypropyl]oxy}-4-hydroxyphenyl)acetamide
[0550] N-{5-chloro-4-methoxy-2-[((2S)-oxiran-2-yl)methoxy]phenyl}acetamide (1g) and 5-fluoro-3H- A solution of spiro[1-benzofuran-2,4'-piperidine] (0.75 g) in ethanol (20 ml) was heated at 80° C. for 1 h, then concentrated in vacuo. The residue was redissolved in dichloromethane, then boron tribromide (1.0M solution in dichloromethane, 3.6ml) was added dropwise. The reaction mixture was stirred at 30 °C for 1 h. Then add the second batch of BBr 3 (1.0M solution in dichloromethane, 3.6ml). The mixture was stirred at 30 °C for 18 h. The reaction was quenched with methanol (20ml) then concentrated in vacuo. The residue was redissolved in dichloromethane (10ml) an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com