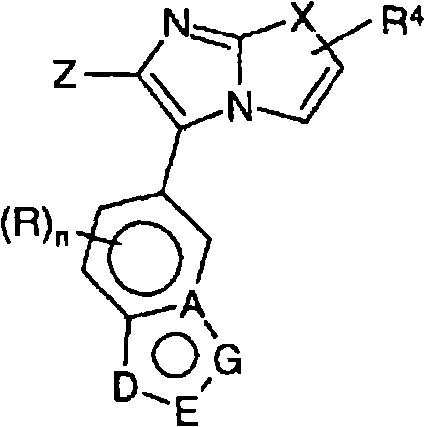
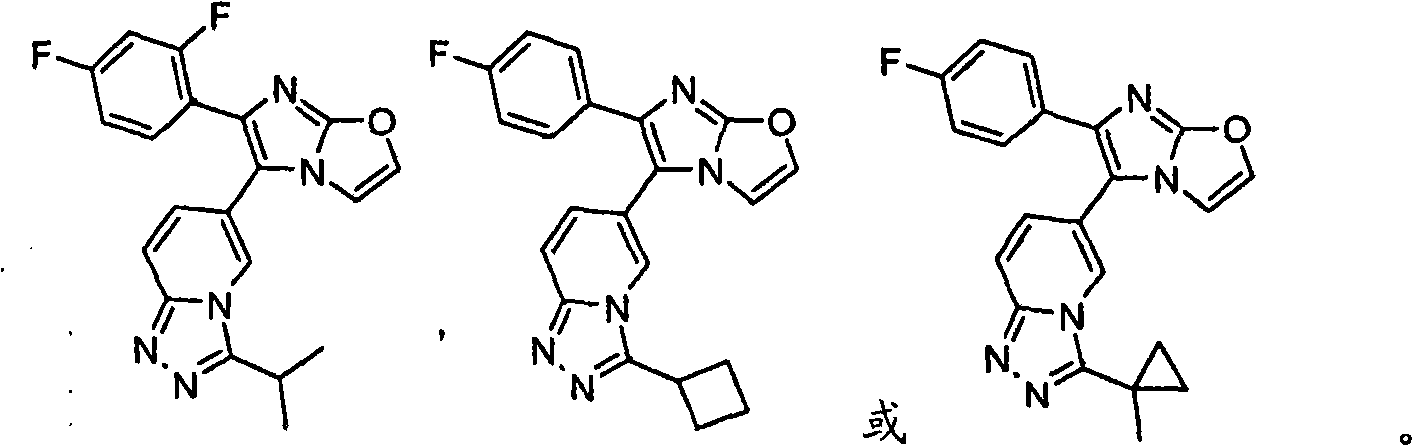
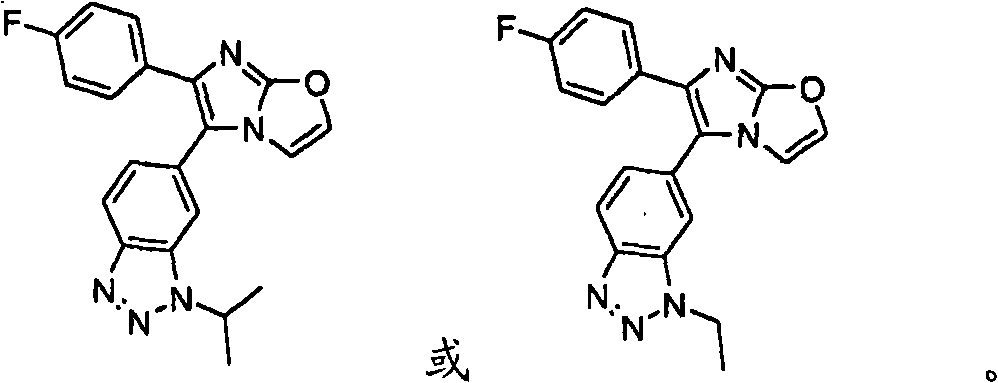
Novel imidazothiazoles and imidazoxazoles
An alkyl and compound technology, applied in the field of novel imidazothiazoles and imidazoxazoles, can solve the problems of excessive and uncontrolled expression of pro-inflammatory cytokines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0215] In the preparation of capsules, 10 parts by weight of the active compound and 240 parts by weight of lactose can be dispersed and blended. The mixture is then filled into hard gelatin capsules, each capsule containing a unit dose or part of a unit dose of the active compound.
[0216] b) Tablet
[0217] Tablets can be prepared from, for example, the following components.
[0218] Parts by weight
[0219] Active compound 10
[0220] Lactose 190
[0221] Corn starch 22
[0222] Polyvinylpyrrolidone 10
[0224] The active compound, lactose and part of the starch are dispersed and blended, and the obtained mixture is granulated with an ethanol solution of polyvinylpyrrolidone. The dry granules are mixed with magnesium stearate and the remaining starch. The mixture is then compressed in a tablet press to obtain tablets each containing a unit dose or part of a unit dose of active compound.
[0225] c) Enteric-coated tablets
[0226] Tablets can be pr...
Embodiment
[0496] Example #I.1.1: 5-(3-Cyclobutyl-[1,2,4]triazolo[4,3-a]pyridin-6-yl)-6-(2,4,5-tri Fluorophenyl)imidazo[2,1-b]oxazole
[0497]
[0498] Add 5-(6-hydrazinopyridin-3-yl)-6-(2,4,5-trifluorophenyl)imidazo[2,1-b]oxazole (0.30g, 0.87) to the round bottom flask mmol; start with preparation #O.1 and NIS with A, use 2-fluoropyridine-5-boronic acid [Asymchem] in C, prepare with hydrazine in D) and cyclobutanecarboxylic acid chloride (1.5mL, 13mmol) . The reaction mixture was heated to about 100°C for about 1 hour. The reaction mixture was diluted with heptane and filtered. The solid was dissolved in DCM (50mL) and washed with saturated aqueous NaHCO 3 (50mL) Wash the organic layer with MgSO 4 Dry, filter and concentrate under reduced pressure. The crude solid was purified by flash chromatography (silica gel; DCM / MeOH gradient from 1:0 to 19:1) to obtain the title compound (0.28 g, 78%): LC / MS (Table 1, Method a) R t = 2.26 minutes; MS m / z: 410.1 (M+H) + .
[0499] Table I.1.1: Using ...
Embodiment P11
[0651] Example P.1.1: 1-(3-(6-(6-(4-fluorophenyl)imidazo[2,1-b]oxazol-5-yl)-[1,2,4]triazole And [4,3-a]pyridin-3-yl)pyrrolidin-1-yl)ethanone
[0652]
[0653] Containing 6-(4-fluorophenyl)-5-(3-(pyrrolidin-3-yl)-[1,2,4]triazolo[4,3-a]pyridin-6-yl)imidazole And [2,1-b]oxazole (0.100g, 0.26mmol; Example #M.1.1.3) in DCM suspension (5mL) was added TEA (0.04mL, 0.26mmol) and acetyl chloride (0.02mL , 0.26mmol). The reaction mixture was allowed to stir for approximately 16 hours at ambient temperature. The crude reaction mixture was purified by flash chromatography (silica gel; DCM / MeOH gradient from 99:1 to 90:10) to give the title compound (0.02 g, 17%). LC / MS (Table 1, Method a) R t = 1.79 minutes; MS m / z: 431.1 (M+H) + .
[0654] Table P.1: Using general method P from 6-(4-fluorophenyl)-5-(3-(pyrrolidin-3-yl)-[1,2,4]triazolo[4,3-a ]Pyridin-6-yl)imidazo[2,1-b]oxazole [Example#M1.1.3] Preparation example
[0655]
Acylating agent
Product
Example#...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com