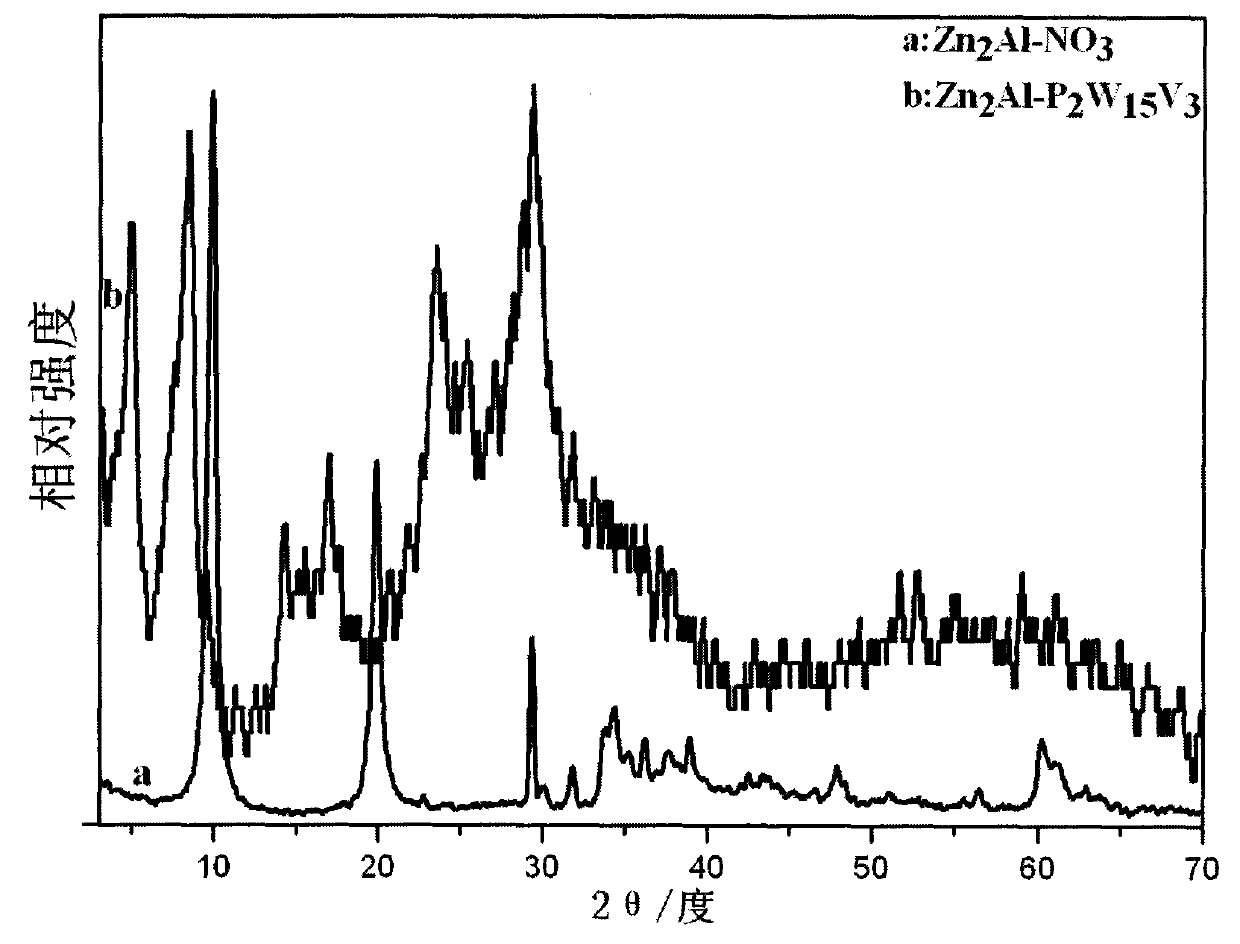
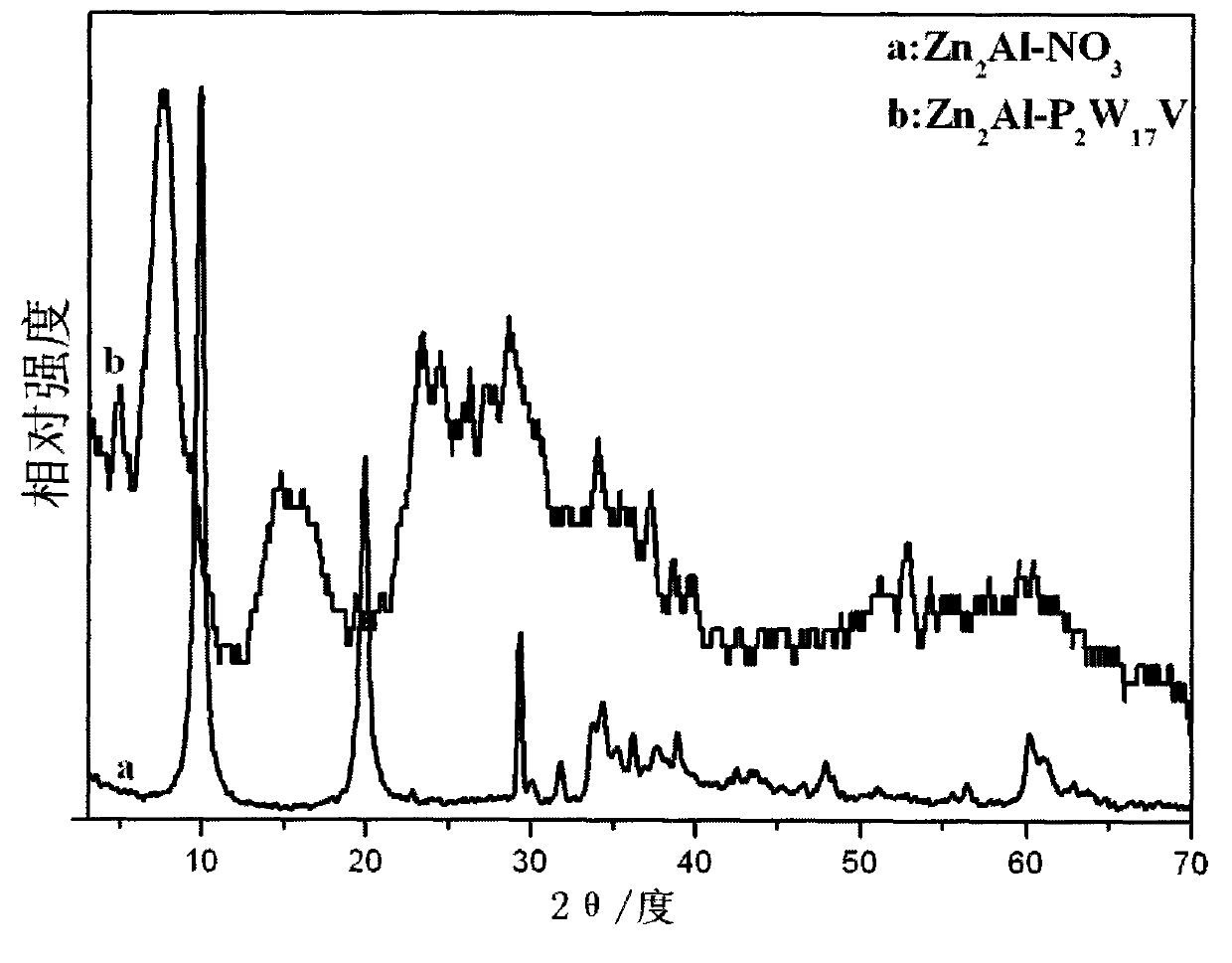
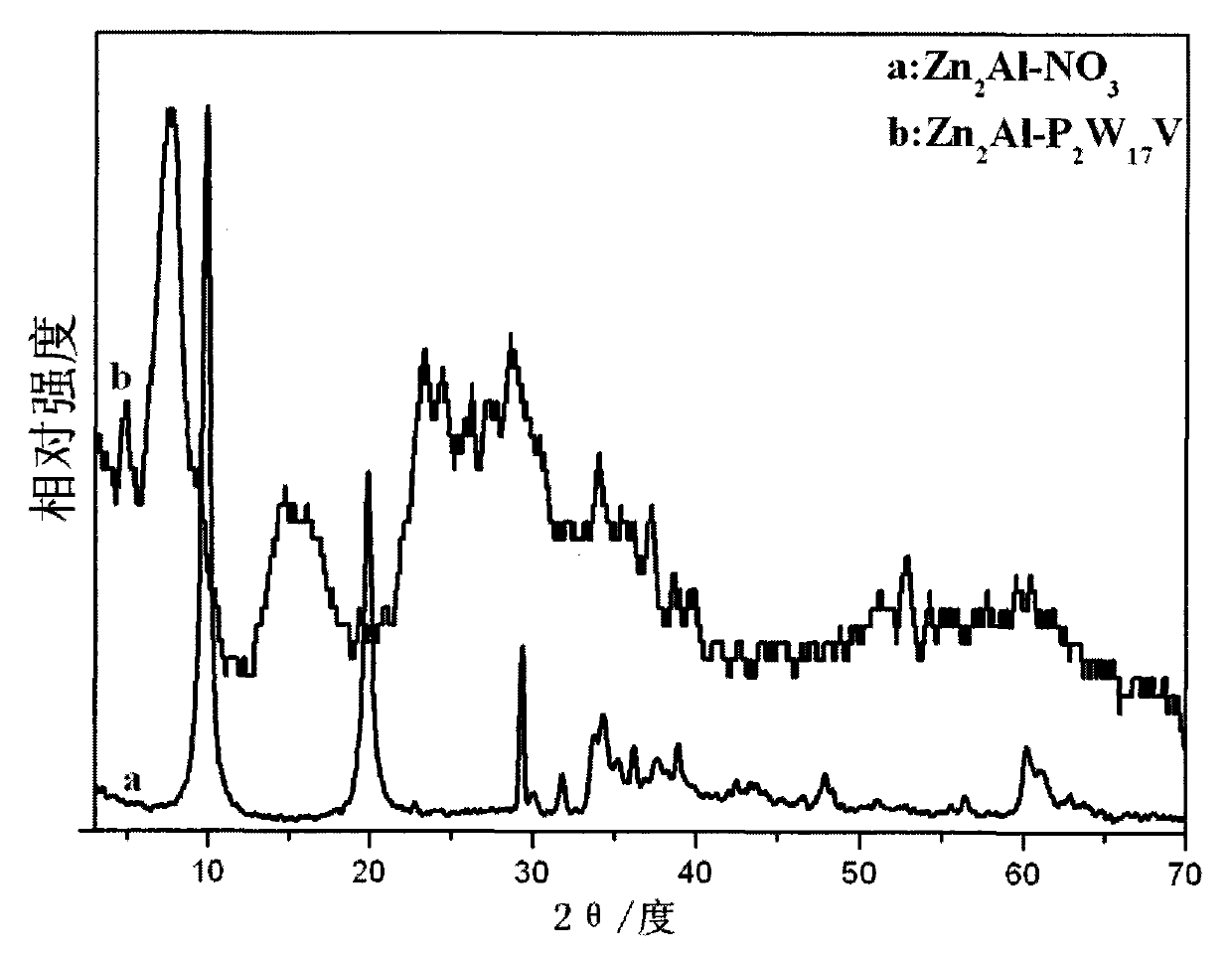
Novel Dawson polyacid intercalation hydrotalcite composite material and method of preparing the same
A composite material and hydrotalcite technology, applied in chemical instruments and methods, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve the problems of Dawson polyacid molecules intercalated into hydrotalcite layers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] a. Take 0.06mol Zn(NO 3 ) 2 ·6H 2 O, 0.03mol Al(NO 3 ) 3 9H 2 O, 0.053mol NaNO 3 Mix and dissolve in 80ml deCO 2 Dissolve 0.133mol NaOH in 50ml of deionized water 2 in deionized water, N 2 Slowly drop the lye into the mixed salt solution under protection, stir vigorously, stop the drop when the pH is 6.0, transfer the slurry to an autoclave, age at 100°C for 18h, and then centrifuge and wash to obtain Zn 2 Al-NO 3 Hydrotalcite precursor;
[0024] b. Take 2.5g Zn 2 Al-NO 3 Hydrotalcite precursor dispersed in 50ml deCO 2 dilute nitric acid to adjust the pH value to 5.5 after swelling in deionized water for 5 hours;
[0025] c. Add 3.09mol of 85% H 3 PO 4 Added to 0.76mol of Na 2 WO 4 2H 2 O solution, heated to reflux for 8h, after cooling, add 100g of NH 4 Cl, the obtained precipitate was dissolved in water to form a solution, and 100 g of NH 4 Cl, the obtained precipitate was dissolved in water again to form a solution, stood still for 5d, filtered, t...
Embodiment 2
[0028] a. Take 0.06mol Zn(NO 3 ) 2 ·6H 2 O, 0.03mol Al(NO 3 ) 3 9H 2 O, 0.053mol NaNO 3 Mix and dissolve in 80ml deCO 2 Dissolve 0.133mol NaOH in 50ml of deionized water 2 in deionized water, N 2 Slowly drop the lye into the mixed salt solution under protection, stir vigorously, stop the drop when the pH is 6.0, transfer the slurry to an autoclave, age at 100°C for 18h, and then centrifuge and wash to obtain Zn 2 Al-NO 3 Hydrotalcite precursor;
[0029] b. Take 2.5g Zn 2 Al-NO 3 Hydrotalcite precursor dispersed in 50ml deCO 2 dilute nitric acid to adjust the pH value to 4.5 after swelling in deionized water for 10 hours;
[0030] c. Add 32.8mmol of NaVO 3 Dissolve in water, add 6M HCl to it, and immediately present a yellow solution, then add 10.7mmol of Na 12 [α-P 2 W 15 o 56 ]·24H 2 After stirring for 10 minutes, 1350 mmol of KCl was added, and an orange-yellow turbid liquid appeared, which was filtered to obtain an orange-yellow solid, which was mixed wit...
Embodiment 3
[0033] a. Take 0.06mol Zn(NO 3 ) 2 ·6H 2 O, 0.03mol Al(NO 3 ) 3 9H 2 O, 0.053mol NaNO 3 Mix and dissolve in 80ml deCO 2 Dissolve 0.133mol NaOH in 50ml of deionized water 2 in deionized water, N 2 Slowly drop the lye into the mixed salt solution under protection, stir vigorously, stop the drop when the pH is 6.0, transfer the slurry to an autoclave, age at 100°C for 18h, and then centrifuge and wash to obtain Zn 2 Al-NO 3 Hydrotalcite precursor;
[0034] b. Take 2.5g Zn 2 Al-NO 3 Hydrotalcite precursor dispersed in 50ml deCO 2dilute nitric acid to adjust the pH value to 5 after swelling in deionized water for 20 hours;
[0035] c. Add 0.5M NaVO to 0.5M hydrochloric acid 3 , continue to join K 10 [α 2 -P 2 W 17 o 61 ]·20H 2 O, after complete dissolution, add KCl, filter the resulting precipitate, dissolve it in 6ml of 0.1M hydrochloric acid and recrystallize to obtain Dawson polyacid 1-K 7 P 2 VW 17 o 62 18H 2 O;
[0036] d. Take 7.43g 1-K 7 P 2 VW 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com