The rna, recombinant and its application for inhibiting human ferroportin
A technology of ferroportin and recombinants, which is applied in the field of retrovirus interference system and drug development and preparation of iron metabolism-related diseases, can solve problems such as inability to achieve therapeutic effects, achieve treatment of iron metabolism-related diseases, and reduce serum iron levels , the effect of inhibiting expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] According to the human ferroportin (FPN1) sequence (NM_000617.1), the template strand and coding strand were designed. Add BglII and XhoI restriction sites to the 5' end respectively, and send the sequence to Invitrogen Company for synthesis. After screening, the FPN1 interference fragment sequence of the present invention with the most significant interference effect is obtained as follows:
[0058]Template strand (Seq ID No.3):
[0059] 5'-GATCCCC AGACTATAATGATAACACT TTCAAGAG AGTGTTATCA TTATAGTCT TTTTTTA-3'
[0060] Coding chain (Seq ID No.4):
[0061] 5’-AGCTTAAAAA AGACTATAATGATAACACT TCTCTTGA AGTGTTATCATTATAGTCT GGGG-3'
Embodiment 2
[0063] Retro-FPN1i system construction
[0064] 1. Anneal the synthesized FPN1 interference fragments (Seq ID No.3 and Seq ID No.4). Specific steps are as follows:
[0065] 1. Establish an annealing system
[0066] 5ul 10x annealing buffer
[0067] 2nmole template chain Seq ID No.3
[0068] 2nmole coding chain Seq ID No.4
[0069] Make up to 50ul with triple distilled water.
[0070] 2. Put it into the PCR instrument, 95°C for 5 minutes, 75°C for 5 minutes, 55°C for 5 minutes, 42°C for 5 minutes, and 37°C for 15 minutes, then gradually decrease the temperature to room temperature.
[0071] 3. Make 12% 1x TAE polyacrylamide glue, take 10ul of the above annealed product and run the glue for 200V1h. The annealed bands were observed by silver staining. Store returned products at -80°C.
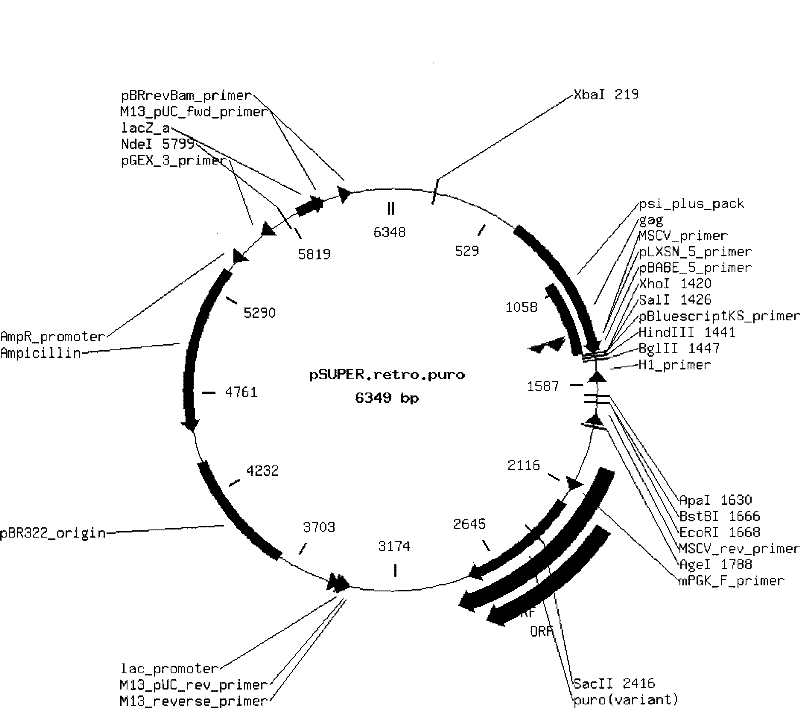
[0072] 3. Enzyme cutting (XbaI and XhoI) retroviral vector ( figure 1 shown), after gel purification and recovery, T4 ligase was used to ligate the annealed product at room temperature f...
Embodiment 3
[0091] Production process of recombinant retrovirus Retro-FPN1i
[0092] 1. Inoculate Phoenix cells 1.5X10 7 cells in a 9cm culture dish (Corning), 37°C, 5% CO 2 Culture overnight;
[0093] 2. Change the fresh medium 20 minutes before transfection;
[0094] 3. Mix 20ul of the above-mentioned Retro-FPN1i with 500ul of DMEM medium and mark it as tube A. Take another 20ul of liposome and mix it with 500ul DMEM medium and mark it as tube B. Mix tube A and tube B, place at room temperature for 30 minutes, gently add the mixture to the culture medium of the 9cm petri dish in step 1, and incubate overnight;
[0095] 5. After 24 hours, add 7.5ml medium to continue culturing for 48 hours;
[0096] 6. Collect the cell supernatant and filter it with a 0.45um filter. The cells can be expanded and cultured, and the virus supernatant can be collected continuously.
[0097] 7. Measure the titer of the virus supernatant, adjust the cell titer to 1000VP / ml with PH8.0 Tris-HCl.
[0098] 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com