Helicobacter pylori viable bacteria carrier vaccine and special recombination bacteria thereof
A technology of Helicobacter pylori and recombinant bacteria, applied in the direction of recombinant DNA technology, the use of vectors to introduce foreign genetic material, bacteria, etc., can solve the problems of less than 20% cure rate, side effects of drugs, poor patient compliance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, preparation and effect of recombinant Shigella flexneri and Helicobacter pylori live bacteria vector vaccine
[0035] 1. Preparation of recombinant Shigella flexneri and Helicobacter pylori live bacterial vector vaccines
[0036] 1. Cloning of Helicobacter pylori urease B subunit (ureB) gene
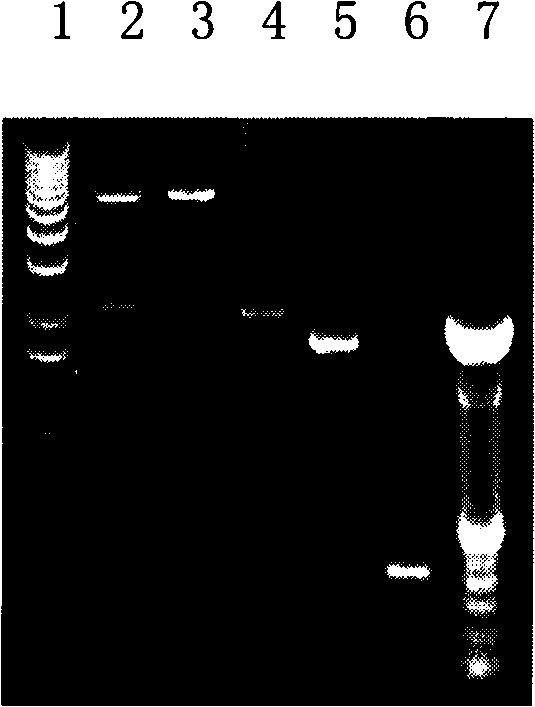
[0037] According to the ureB gene sequence of Helicobacter pylori 26695, primers were designed with PCR primer design and analysis software (P1: 5'-TGCGAGCTCAAAAAGATTAGCAGAAAA-3'; P2: 5'-GGCTGCAGGAAAATGCTAAAGAGTTG-3'), and Helicobacter pylori 26695 (purchased from Genomic DNA from the Chinese Center for Disease Control and Prevention) was used as a template, and the high-fidelity Pyrobest DNA polymerase (purchased from Takala Company) was used to carry out PCR amplification to obtain the H.pylori ureB gene, such as figure 1 shown.
[0038] After purification, the ureB gene fragment amplified by PCR was digested with SacI and PstI (purchased from NEB Company) and liga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com