Preparation and applications of RGD-fatty amine series compound as tumor targeting vector material
A compound and targeting technology, applied in the preparation of targeting carrier materials, the application field of drug carriers in the preparation of targeted drug delivery preparations, can solve the problems of ordinary nano-drug delivery systems not being targeted, and achieve excellent target The effect of tropism, strong stability, and not easy to be degraded by enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
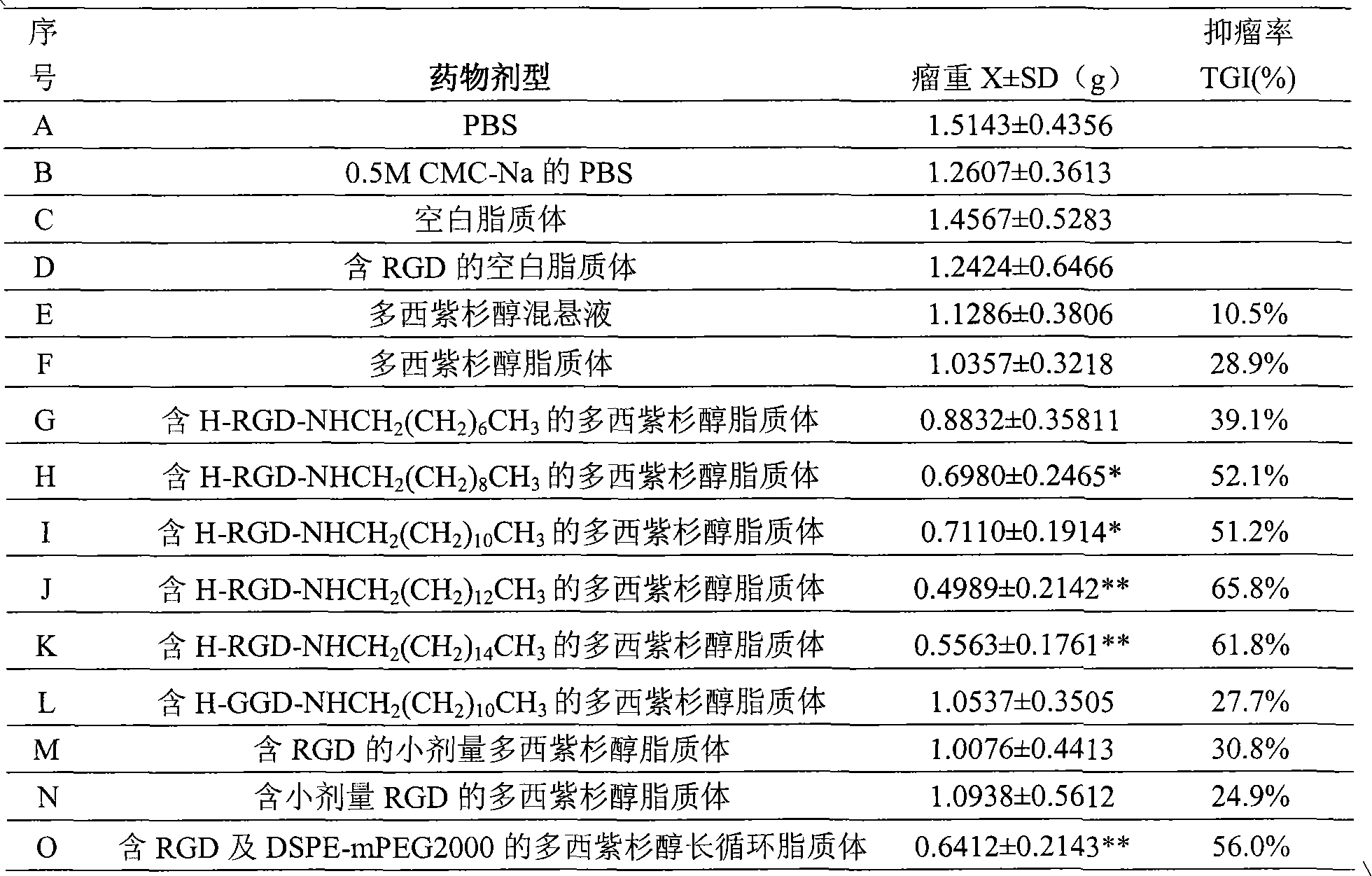
Examples
Embodiment 1
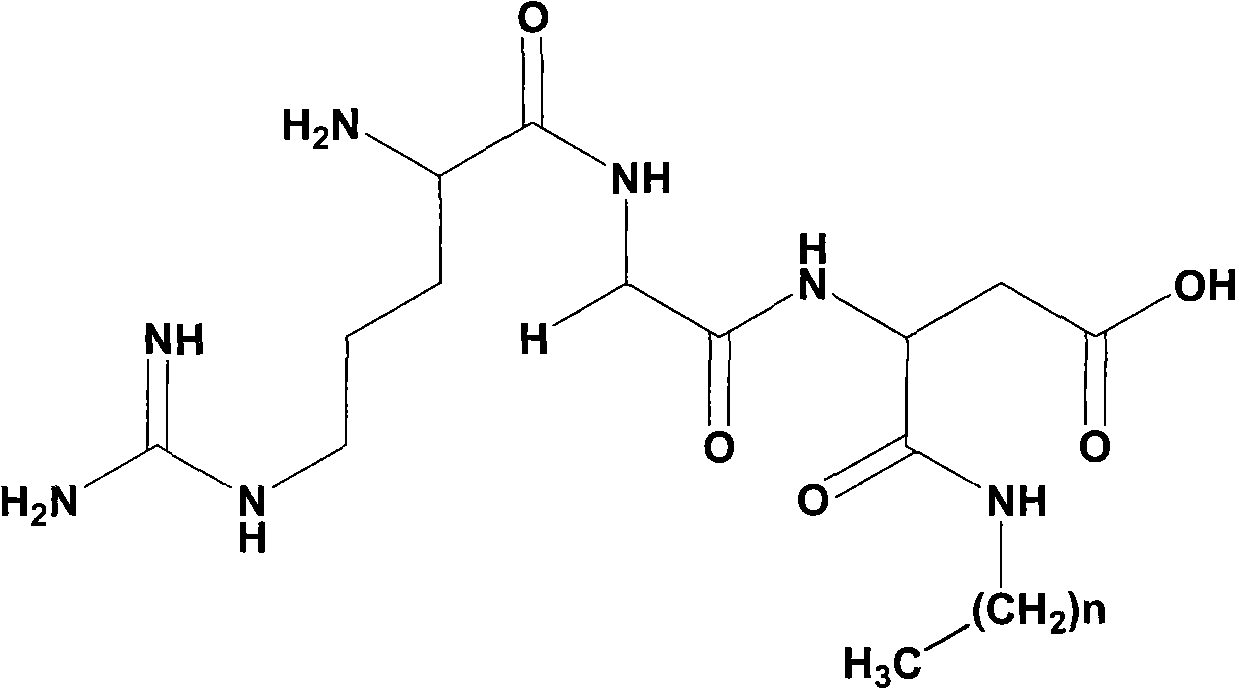
[0038] Example 1 Boc-Arg (NO 2 )-Gly-OMe Preparation
[0039] 1.595g (5.0mmol) Boc-Arg (NO 2)-OH was dissolved in 20ml of anhydrous DMF, and under ice-cooling conditions, 0.675g (5mmol) of N-hydroxybenzotriazole (HOBt) was added and completely dissolved. After 10 minutes 1.236 g (6 mmol) of dicyclohexylcarbodiimide (DCC) were added. Obtain reaction liquid (I), stand-by. Dissolve 0.628g (5.0mmol) HCL·Gly-OMe (5mmol) in 20ml THF under ice-cooling, then add 1ml N-methylmorpholine (NMM) to adjust the pH to 8-9. Stir for 35 minutes to obtain the reaction solution (II), which is ready for use. The reaction solution (I) was added to the reaction solution (II) under the ice bath, first stirred under the ice bath for 1 h, then stirred at room temperature for 12 h, TLC (chloroform / methanol, 10:1) showed that Boc-Arg (NO 2 )-OH disappears. Dicyclohexylurea (DCU) was filtered off, and the filtrate was blown off of DMF. The residue was dissolved with 50 ml of ethyl acetate. The res...
Embodiment 2
[0040] Example 2 Boc-Arg (NO 2 )-Gly-OH preparation
[0041] 0.780g (2.0mmol) Boc-Arg (NO 2 )-Gly-OMe was dissolved in 10 ml methanol. Adjust pH to 10-11 with NaOH (2N) aqueous solution under ice bath and stir for 4h, TLC (chloroform / methanol, 10:1) showed that Boc-Arg (NO 2 )-Gly-OMe disappears. The reaction mixture was saturated with KHSO 4 Adjust the pH to 7, and concentrate under reduced pressure to remove methanol. The residue was washed with saturated KHSO 4 Adjust the pH to 2, and extract with ethyl acetate (30ml×3). The combined ethyl acetate phases were washed with saturated NaCl aqueous solution to neutral, anhydrous NaCl 2 SO 4 dry. After filtration, the filtrate was concentrated to dryness under reduced pressure to afford 0.591 g (78.7%) of the title compound as a white solid. (ESI-MS(m / z): 375.3[M] - )
Embodiment 3
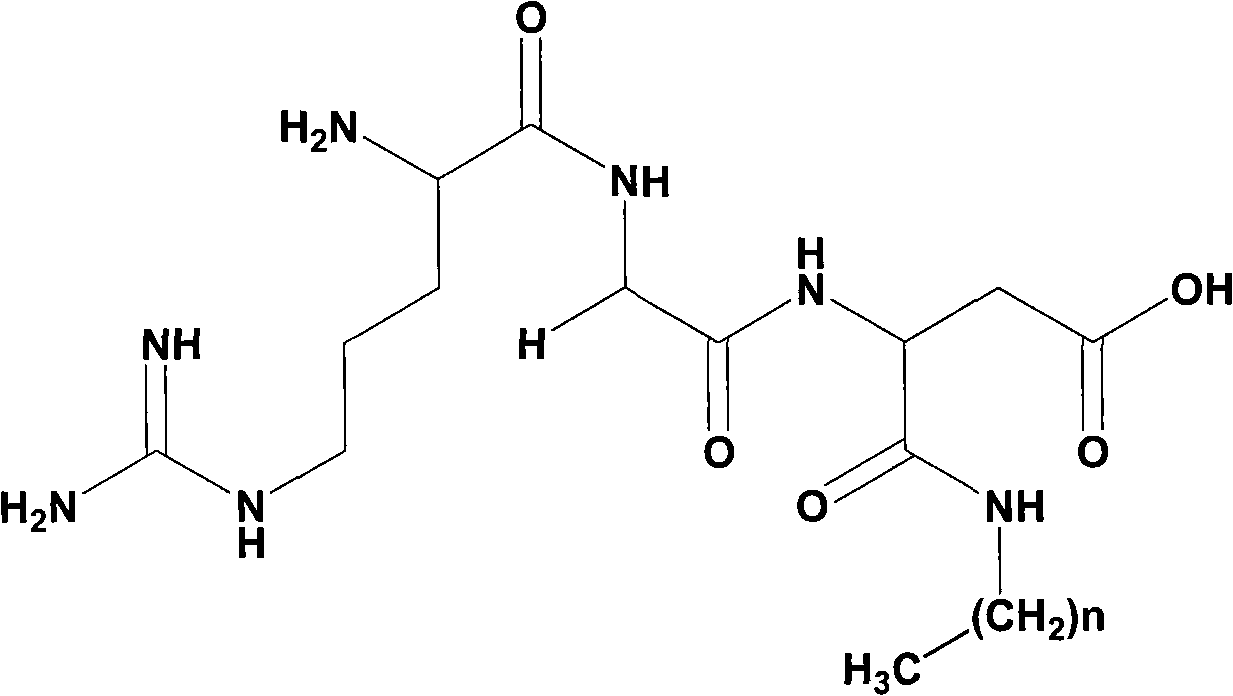
[0043] 1) Boc-Asp(OBzl)-NHCH 2 (CH 2 ) 6 CH 3 preparation of
[0044] According to Boc-Arg (NO 2 ) The preparation method of Gly-OMe, 1.615g (5mmol) Boc-Asp (OBzl)-OH and 0.541g (4.5mmol) CH 3 (CH 2 ) 6 CH 2 NH 2 1.918 g (98.2%) of a crude beige solid was obtained, and purified by a silica gel column to obtain 1192 g (61.0%) of the title compound as a white solid. (ESI-MS(m / z): 435.7[M] + )
[0045] 2) HCl Asp(OBzl)-NHCH 2 (CH 2 ) 6 CH 3 preparation of
[0046] 0.868g (2.0mmol) Boc-Asp(OBzl)-NHCH 2 (CH 2 ) 6 CH 3 Dissolve in 10ml 4mol / l hydrogen chloride-ethyl acetate solution, stir at room temperature for 4 hours, TLC (chloroform / methanol, 5:1) shows that the raw material point disappears, concentrate under reduced pressure to remove ethyl acetate, add a small amount of ether to the residue repeatedly to reduce Pump dry under pressure to remove hydrogen chloride gas. Finally, a small amount of diethyl ether was added to triturate the residue to obtain 0.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com