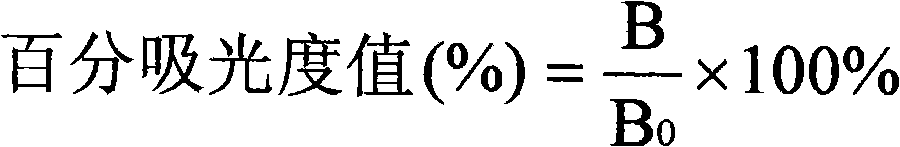Furaltadone metabolite detection kit
A technology for detecting kits and furaltadone, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that the accuracy, sensitivity, and specificity cannot be better than imported kits, and achieve the effect of good performance and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] d. Preparation and purification of monoclonal antibody: Using in vivo induction method, Balb / c mice (8 weeks old) were intraperitoneally injected with 0.5ml of sterilized paraffin oil per mouse, and 5×10 hybridoma cells were injected intraperitoneally after 7-14 days 5 -10 6 Ascites collected 7-10 days later, purified by octanoic acid-saturated ammonium sulfate method, divided into small bottles, and stored at -20°C;
[0049] e. Antibody freeze-dried powder can dry the ascites at 37°C and store it at -20°C;
[0050] f. Antibody working solution is to dilute the antibody at a concentration of 0.02-0.08 μg / ml with antibody diluent.
[0051] (2) The steps for preparing the polyclonal antibody of furaltadone metabolite derivatives are:
[0052] New Zealand white rabbits were adopted as immunized animals, and the immunogen (the conjugate of furaltadone metabolite derivative hapten and keyhole limpet hemocyanin) was immunized at 1 mg / kg. Mix complete emulsifier with Freund...
Embodiment 1
[0112] Example 1 Preparation of ELISA kit components for detecting furaltadone metabolites
[0113] 1. Antigen Synthesis
[0114] a. Synthesis of Coating Progen
[0115] The furaltadone metabolite is synthesized by the o-nitrobenzaldehyde derivative method to synthesize the furaltadone metabolite derivative hapten, and then the hapten is obtained by coupling the hapten with the bovine gamma globulin carrier protein by the active ester method through a diazotization reaction.
[0116] b. Synthesis of Immunogen
[0117] The furaltadone metabolite was synthesized by the o-nitrobenzaldehyde derivative method to synthesize the furaltadone metabolite derivative hapten, and then the hapten was coupled with the keyhole limpet hemocyanin carrier protein by the active ester method through the diazotization reaction. Get it.
[0118] 2. Preparation of mouse monoclonal antibody against furaltadone metabolite derivatives
[0119] a. Animal immunity
[0120] use. Balb / c mice were used...
Embodiment 2
[0131] Example 2 The formation of an enzyme-linked immunosorbent assay kit for detecting furaltadone metabolites
[0132] An enzyme-linked immunosorbent assay kit for detecting furaltadone metabolites was constructed to include the following components:
[0133] (1) an enzyme-linked plate coated with furaltadone metabolite derivative antigen;
[0134] (2) specific mouse antibody of furaltadone metabolite derivatives labeled with horseradish peroxidase;
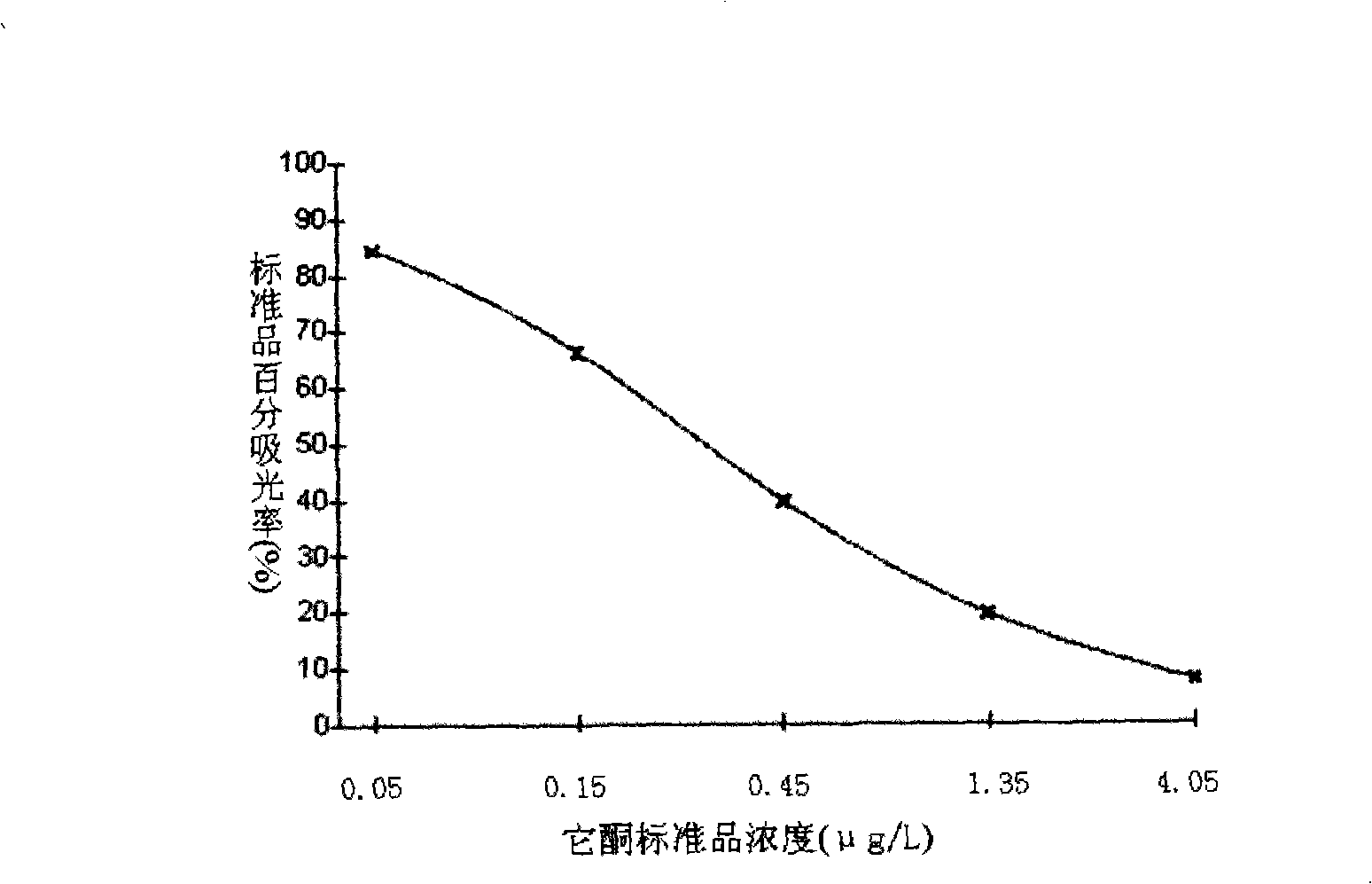
[0135] (3) 6 bottles of furaltadone metabolite standard solution, the concentrations were 0μg / L, 0.05μg / L, 0.15μg / L, 0.45μg / L, 1.35g / L, 4.05μg / L;
[0136] (4) Substrate chromogenic solution hydrogen peroxide and tetramethylbenzidine sulfate mixed solution;
[0137] (5) The stop solution is 2mol / L sulfuric acid buffer;
[0138] (6) The concentrated washing liquid is deionized water or ultrapure water;
[0139] (7) Antibody diluent is pH 7.4, 0.08mol / L phosphate buffer containing 0.3% gelatin, 5‰ Tween-80 and 5‰ methanol.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com