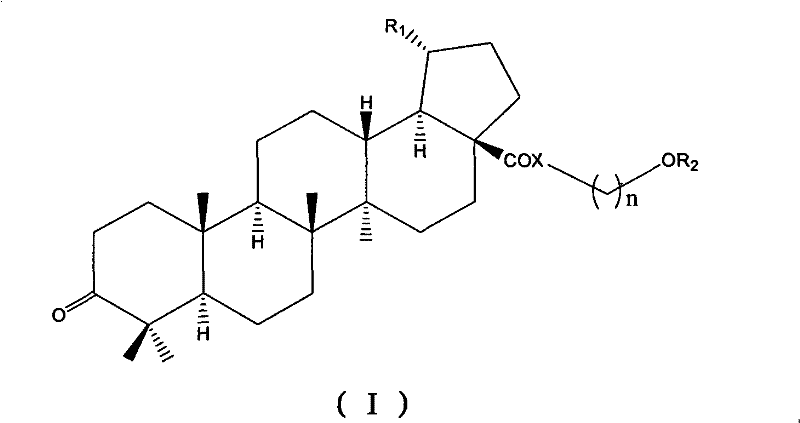
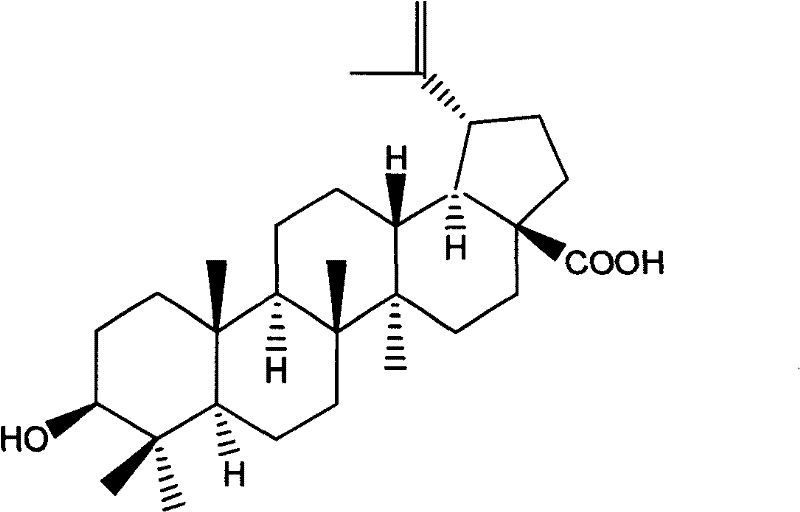
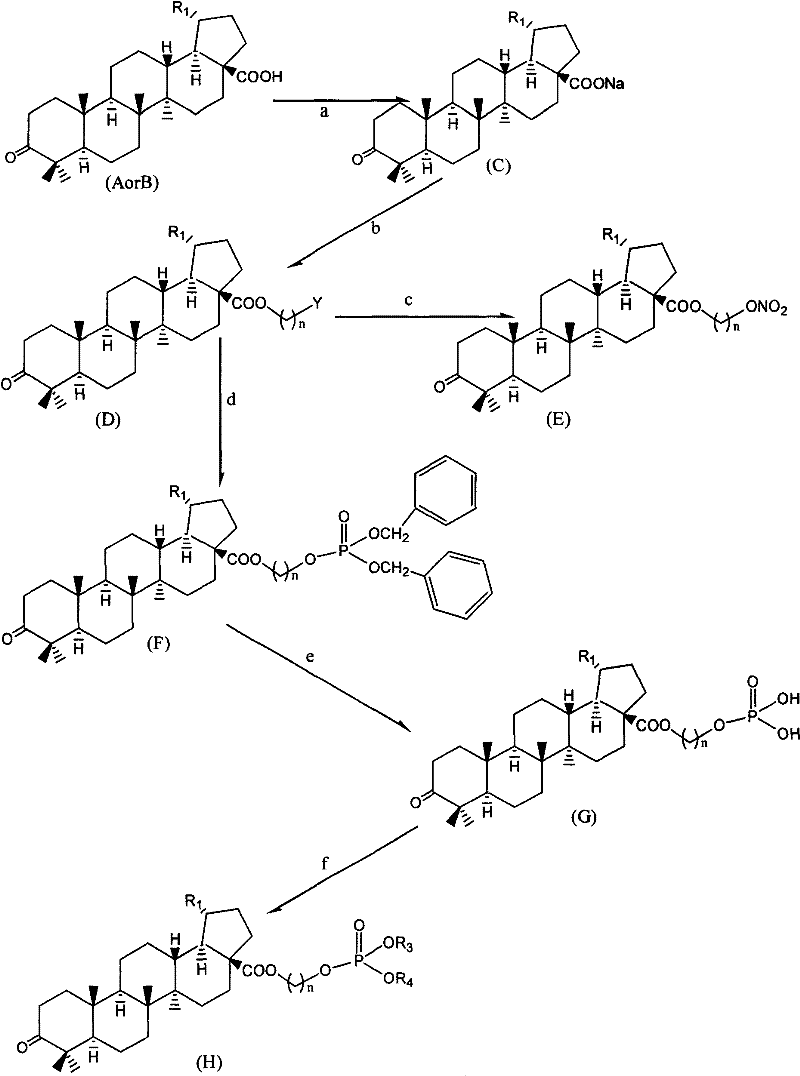
Betulinic acid analogue and preparation method and application thereof
A technology of betulinic acid and betulinamide, applied in the field of medicine, can solve the problems of poor water solubility, anti-tumor activity to be improved, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0160] Example 1: 3-deoxy-3-oxo-betulinic acid (3-nitrate) propyl ester (HK-101)
[0161] a. Add 18.2g of 3-deoxy-3-oxo-betulinic acid (compound A) and 250ml of anhydrous methanol to the reaction flask, slowly add 2.2g of sodium methoxide under stirring, and keep warm for 1.5 hours at room temperature. Then the solvent was evaporated under reduced pressure and dried to obtain off-white sodium salt. The sodium salt can be used in the next step without purification.
[0162] b. In the reaction flask, add 9.5g of 3-deoxy-3-oxo-sodium betulinate, 100ml of dimethylformamide (DMF), and add 6.3g of 1,3-bromochloropropane dropwise under stirring, within 30 minutes After the dropwise addition was completed, the reaction was maintained at 40° C. for 18 hours. Stop the reaction, filter to remove the generated sodium bromide. DMF was evaporated under reduced pressure, 85ml of dichloromethane was added, and the insoluble matter was removed by filtration. Dichloromethane was evaporated ...
Embodiment 2
[0169] Example 2: 3-deoxy-3-oxo-betulinic acid (4-nitrate) butyl ester (HK-102)
[0170] According to the preparation method and process provided in Example 1, the 3-deoxy-3-oxo-betulinic acid (4-bromo) butyl ester obtained in Example 1 (b) is replaced in Example 1 (c) 3-Deoxy-3-oxo-betulinic acid (3-chloro)propyl ester, you can get 3-deoxy-3-oxo-betulinic acid (4-nitrate) butyl ester (HK-102) . HPLC: 99.18%, ESI-MS: 594.4 (M+Na).
Embodiment 3
[0171] Example 3: 3-deoxy-3-oxo-betulinic acid (6-nitrate) hexyl ester (HK-103)
[0172] According to the preparation method and process provided in Example 1, the 3-deoxy-3-oxo-betulinic acid (6-chloro) butylhexyl ester obtained in Example 1 (b) is replaced in Example 1 (c). 3-deoxy-3-oxo-betulinic acid (3-chloro)propyl ester, 3-deoxy-3-oxo-betulinic acid (6-nitrate) hexyl ester (HK-103), HPLC: 99.26%, ESI-MS: 622.4 (M+Na).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com