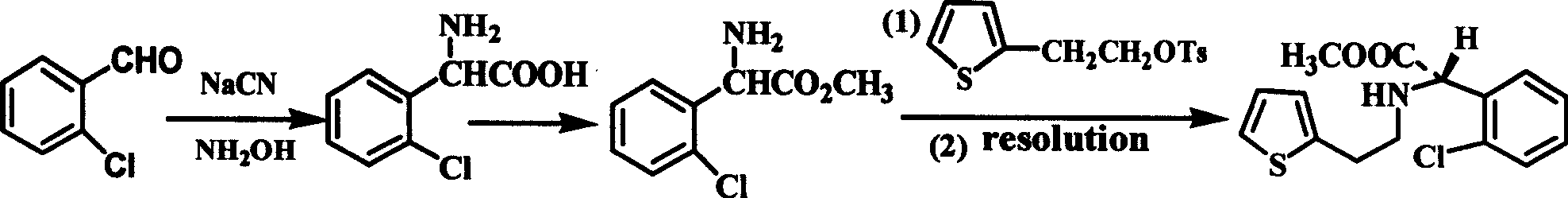
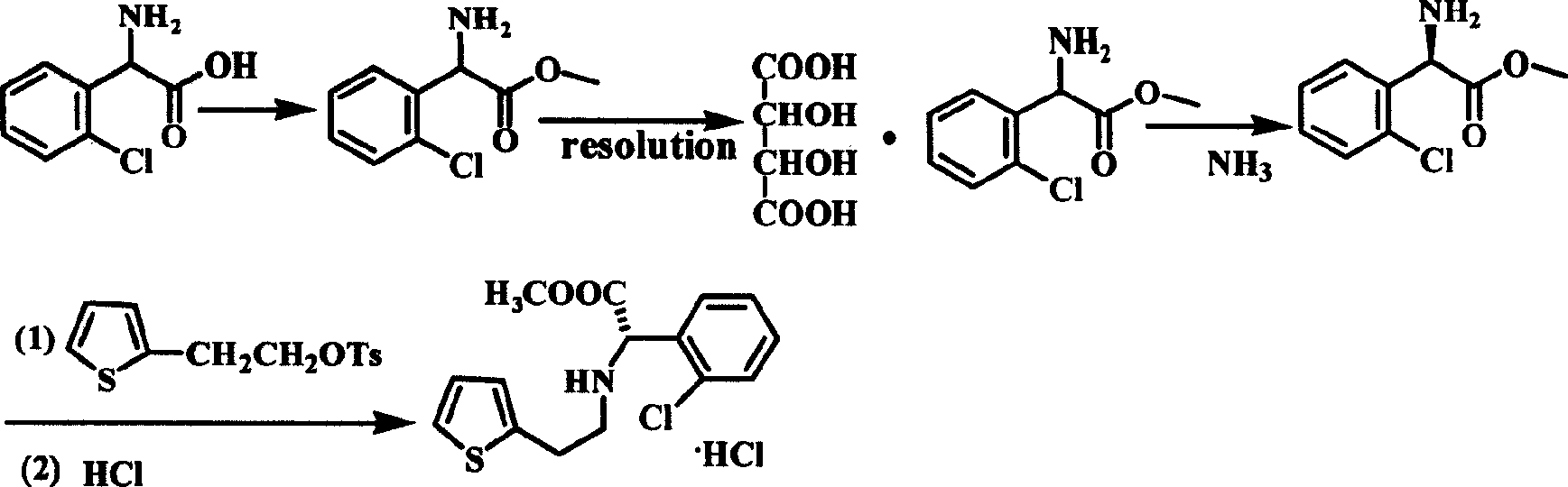
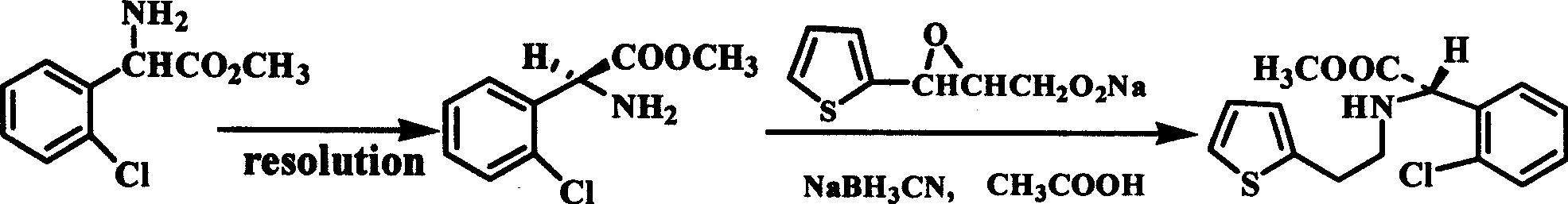Intermediate (S)-2-(2-thiophene ethylamine)(2-chlorphenyl)methyl acetate of clopidogrel and method for preparing salts thereof
A technology of thienylethylamine and o-chlorophenylglycine methyl ester, applied in organic chemistry, chemical instruments and methods, organic chemical methods, etc., can solve the problems of easy racemization and affecting the optical purity of products, and achieve good optical rotation , The effect of shortening the preparation cycle and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 (S)-(+)-methyl o-chlorophenylglycine L-tartrate (5.00g, 0.0143mol) and thiophene-2-ethyl p-toluenesulfonate (5.00g, 0.0177mol) is placed in the three-necked flask, adding K 2 HPO 4 ·3H 2 O (18.50g), react at 97-99°C for 12hrs, after the reaction, add water (50ml) and ethyl acetate (40ml), stir, separate liquids, extract the organic layer, adjust the pH with concentrated hydrochloric acid: between 1.2-1.5, store on ice Water bath cooled and filtered to obtain 4.08g product, mp: 181.6-181.8°C, [α] D 25 =+109.4° (c=1.0, CH 3 OH); Yield: 82.5%. 1 HNMR (D 2O, 500MHz), δ: 7.65~7.51(m, 4H), 7.30(t, J=3.5Hz, 1H), 6.98(t, J=3.5Hz, 2H), 5.75(s, 1H), 3.85(s , 3H), 3.84~3.12(m, 4H) (literature value: mp: 180—182℃, [α] D 25 =+108.5-110°).
[0032] Add 4.00g of the product obtained above, 8ml of methanol, and 26ml of formaldehyde (37%) into a three-necked flask equipped with a stirring and reflux device, and follow the process steps of the patent (US.Pat.No.452959...
Embodiment 2
[0033] Example 2 (S)-(+)-o-chlorophenylglycine methyl ester L-tartrate (5.00g, 0.0143mol) and thiophene-2-ethyl p-toluenesulfonate (5.00g, 0.0177mol) was placed in the three-necked flask, adding Na 2 CO 3 (3.70g), water (1ml), and react at 96-99°C for 11hr. After the reaction, add water (50ml) and ethyl acetate (40ml), stir and separate, extract the organic layer, adjust the pH with concentrated hydrochloric acid: 1.2- Between 1.5, cooling and filtering in an ice-water bath, 4.03g of product was obtained, mp: 180.0-180.2°C, [α] D 25 =+108.7° (c=1.0, CH 3 OH), yield: 81.5%.
Embodiment 3
[0034] Example 3 (S)-(+)-o-chlorophenylglycine methyl ester L-tartrate (5.00g, 0.0143mol) and thiophene-2-ethyl benzenesulfonate (5.00g, 0.0177 mol) was placed in a three-necked flask, and Na was added 2 HPO 4 12H 2 O (13g), Na 2 CO 3 (2.60g), react at 94-99°C for 13hr, after the reaction, add water (50ml) and ethyl acetate (40ml), stir, separate liquids, extract the organic layer, adjust the pH between 1.2-1.5 with concentrated hydrochloric acid, store on ice Water bath cooled and filtered to obtain 4.00g product, mp: 179.8-180.1°C, [α] D 25 =+107.5° (c=1.0, CH 3 OH), yield: 80.9%.
[0035] Adopt the synthetic route similar to Example 1 to prepare (S)-2-(2-thienylethylamino)(2-chlorophenyl)methyl acetate and its salts under different process conditions, the results are shown in Table 1:
[0036] Table 1
[0037]
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com