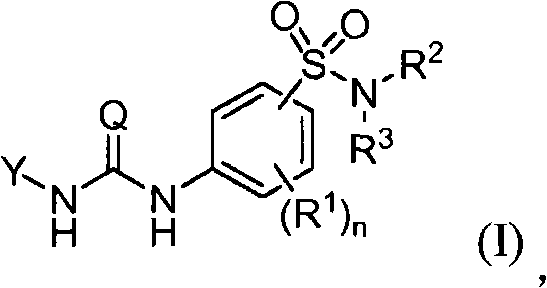
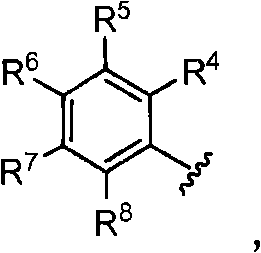
Soluble epoxide hydrolase inhibitors
A compound, cycloalkyl technology, applied in the field of medicinal chemistry, can solve the problem of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0327] In the examples below and throughout the application, the following abbreviations have the following meanings. If not defined otherwise, said terms have their commonly accepted meanings.
[0328] aq. = aqueous solution
[0329] brs = broad singlet
[0330] d = doublet
[0331] DCM = dichloromethane
[0332] DMAP = dimethylaminopyridine
[0333] DMF = dimethylformamide
[0334] DMSO = dimethyl sulfoxide
[0335] EtOAc = ethyl acetate
[0336] g = gram
[0337] LCMS = Liquid Chromatography Mass Spectrometry
[0338] m = multiplet
[0339] MHz = megahertz
[0340] mL = milliliter
[0341] m.p. = melting point
[0342] N = equivalent
[0343] RT = room temperature
[0344] s = singlet
[0345] t = triplet
[0346] TEA = Triethylamine
[0347] TLC = thin layer chromatography
example 1
[0349] 4-(3-Adamantan-1-yl-ureido)-benzenesulfonamide (1)
[0350]
[0351] Adamantyl isocyanate (0.07 mL, 0.745 mmol) was added to a stirred solution of 4-aminobenzenesulfonamide (150 mg, 0.850 mmol) in ethanol (15 mL) at room temperature overnight. The precipitated solid was filtered, washed with petroleum ether and pentane and recrystallized in acetone to give the title compound (100 mg, 49%) as a white solid; mp 270-275 °C; LC / MS purity 99.7%; mass: 350[ M+1]; 1 HNMR: (300MHz; DMSO-D 6 )δ: 1.60-2.20 (m, 15H CH 2 ); 7.23-7.65 (4H, ArCH); 9.26 (brs, 2H, NH).
example 2
[0353] 3-(3-Adamantan-1-yl-ureido)-benzenesulfonamide (2)
[0354]
[0355] To a stirred solution of 3-nitrobenzenesulfonamide (300 mg, 0.95 mmol) in methanol (10 mL) was added Pd-C (100 mg) and stirred at room temperature under hydrogen atmosphere overnight. The solution was filtered and the filtrate was concentrated under vacuum to give 3-aminobenzenesulfonamide as a yellow solid (yield 250 mg); mass: 172 [M+1].
[0356] To a stirred solution of 3-aminobenzenesulfonamide (150 mg, 0.872 mmol) in ethanol (15 mL) was added adamantyl isocyanate (0.12 mL, 0.855 mmol) at room temperature overnight. The precipitated solid was filtered, washed with petroleum ether and pentane and recrystallized in acetone to give the title compound (180 mg, 45%) as an off-white solid; mp 158-162 °C; LC / MS purity 99.4%; mass: 360[M+1]; 1 HNMR: (300MHz; DMSO-D 6 )δ: 1.60-2.19 (15H, adamantyl CH 2 ); 7.20-7.45 (4H, ArCH); 9.26 (brs, 2H, NH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com