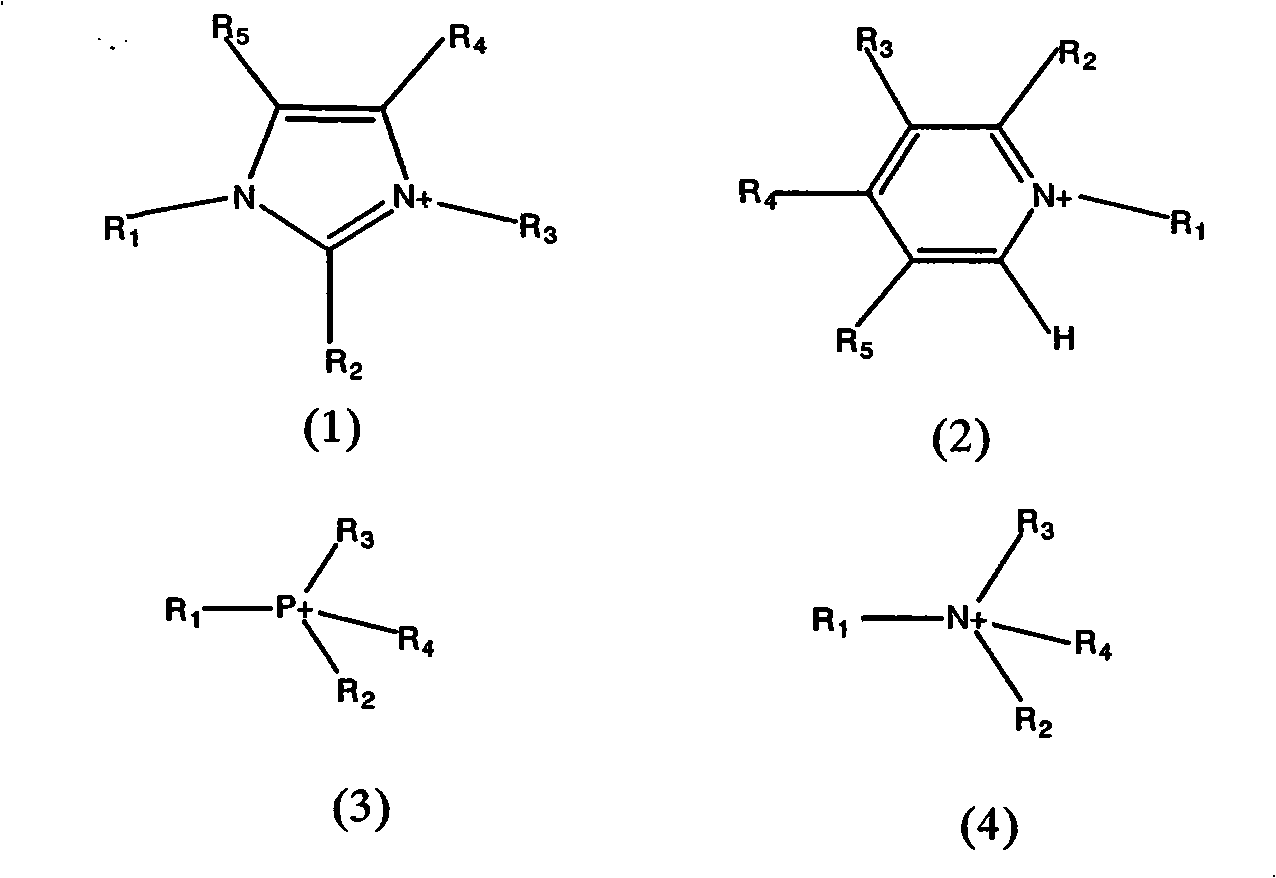
New method for desulfurization by oxidation based on alkaline ionic liquid catalyst
A liquid catalyst, basic ion technology, applied in separation methods, chemical instruments and methods, combustible gas purification, etc., can solve problems such as unreported methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Before the experiment, add 10ml of monoethanolamine and 10ml of basic ionic liquid into the equilibrium reaction kettle, and stir evenly. Repeat vacuuming three times to ensure the vacuum in the kettle. Then pass a certain mass ratio of CH into the kettle 4 / H 2 S mixed gas, under the conditions of pressure 0.10MPa and temperature 303K, air is blown in. Detection after 5 hours: CH 4Essentially not absorbed; hydrogen sulfide is absorbed and catalytically oxidized to elemental sulfur. The absorption rate of hydrogen sulfide is above 95%, and the conversion rate of hydrogen sulfide is above 85%. The catalyst was reused 5 times, and the absorption rate and conversion of hydrogen sulfide remained basically unchanged.
Embodiment 2
[0021] Before the experiment, add 10ml of diethanolamine and 10ml of basic ionic liquid into the equilibrium reaction kettle, and stir evenly. Repeat vacuuming three times to ensure the vacuum in the kettle. Then pass a certain mass ratio of CH into the kettle 4 / H 2 S mixed gas, under the conditions of pressure 0.10MPa and temperature 303K, air is blown in. Detection after 5 hours: CH 4 Essentially not absorbed; hydrogen sulfide is absorbed and catalytically oxidized to elemental sulfur. The absorption rate of hydrogen sulfide is above 95%, and the conversion rate of hydrogen sulfide is above 85%. The catalyst was reused 5 times, and the absorption rate and conversion of hydrogen sulfide remained basically unchanged.
Embodiment 3
[0023] Before the experiment, add 10ml of isobutanolamine and 10ml of basic ionic liquid into the equilibrium reaction kettle, and stir evenly. Repeat vacuuming three times to ensure the vacuum in the kettle. Then pass a certain mass ratio of CH into the kettle 4 / H 2 S mixed gas, under the conditions of pressure 0.10MPa and temperature 303K, air is blown in. Detection after 5 hours: CH 4 Essentially not absorbed; hydrogen sulfide is absorbed and catalytically oxidized to elemental sulfur. The absorption rate of hydrogen sulfide is above 95%, and the conversion rate of hydrogen sulfide is above 85%. The catalyst was reused 5 times, and the absorption rate and conversion of hydrogen sulfide remained basically unchanged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com