Process for preparing nanocrystalline mixed metal oxides and nanocrystalline mixed metal oxides obtained using the process
A nanocrystal, mixed metal technology, applied in metal/metal oxide/metal hydroxide catalysts, oxygen/ozone/oxide/hydroxide, chemical instruments and methods, etc. Crystal particles unfavorable agglomeration and other problems, to achieve the effect of enhanced reactivity, increased BET surface area, conversion rate and selectivity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] LaMnO 3 preparation of
[0063] 2.5112g Mn(NO 3 ) 2 4H 2 O and 4.3302g La(NO 3 ) 2 ·6H 2 O and 4.2028 g of citric acid were dissolved together in 30 ml of water at a temperature of 80°C and slowly reheated to 150°C.
[0064] The resulting viscous solution was then introduced in atomized form through a Schenk distributor into the previously described reactor. The residence time of the solution in the reactor was about 700 milliseconds. The temperature was set at 270°C.
[0065] Then 6.1g LaMnO 3 (corresponding to 95% yield) was removed from the reactor with a BET surface area of 175m 2 / g.
Embodiment 2
[0067] La 0.5 Sr 0.5 MnO 3 preparation of
[0068] 1.07g Sr(NO 3 ) 2 , 2.60g Mn(NO 3 )·4H 2 O, 2.22g La(NO 3 ) 2 ·6H 2 O and 4.20g of citric acid were dissolved in 30ml of water at 80°C.
[0069] The solution was introduced into the reactor as in Example 1 via a Schenk distributor. The residence time of the powder in the reactor was likewise about 700 milliseconds, and the temperature of the reactor was 200°C. La 0.5 Sr 0.5 MnO 3 The yield of 4.5g, BET surface area is 185m 2 / g.
Embodiment 3
[0071] In the process of oxidizing CO, the La obtained in Example 2 0.5 Sr 0.5 MnO 3 Compared with La prepared by hydroxide precipitation method and subsequent calcination 0.5 Sr 0.5 MnO 3 Do a test comparison.
[0072] In each case 100 mg La 0.5 Sr 0.5 MnO 3 Mixed with 500mg of silica sand, the flow rate allowed for the reaction is 35ml per minute (the CO in the synthesis gas is 802ppm). Powder is not activated.
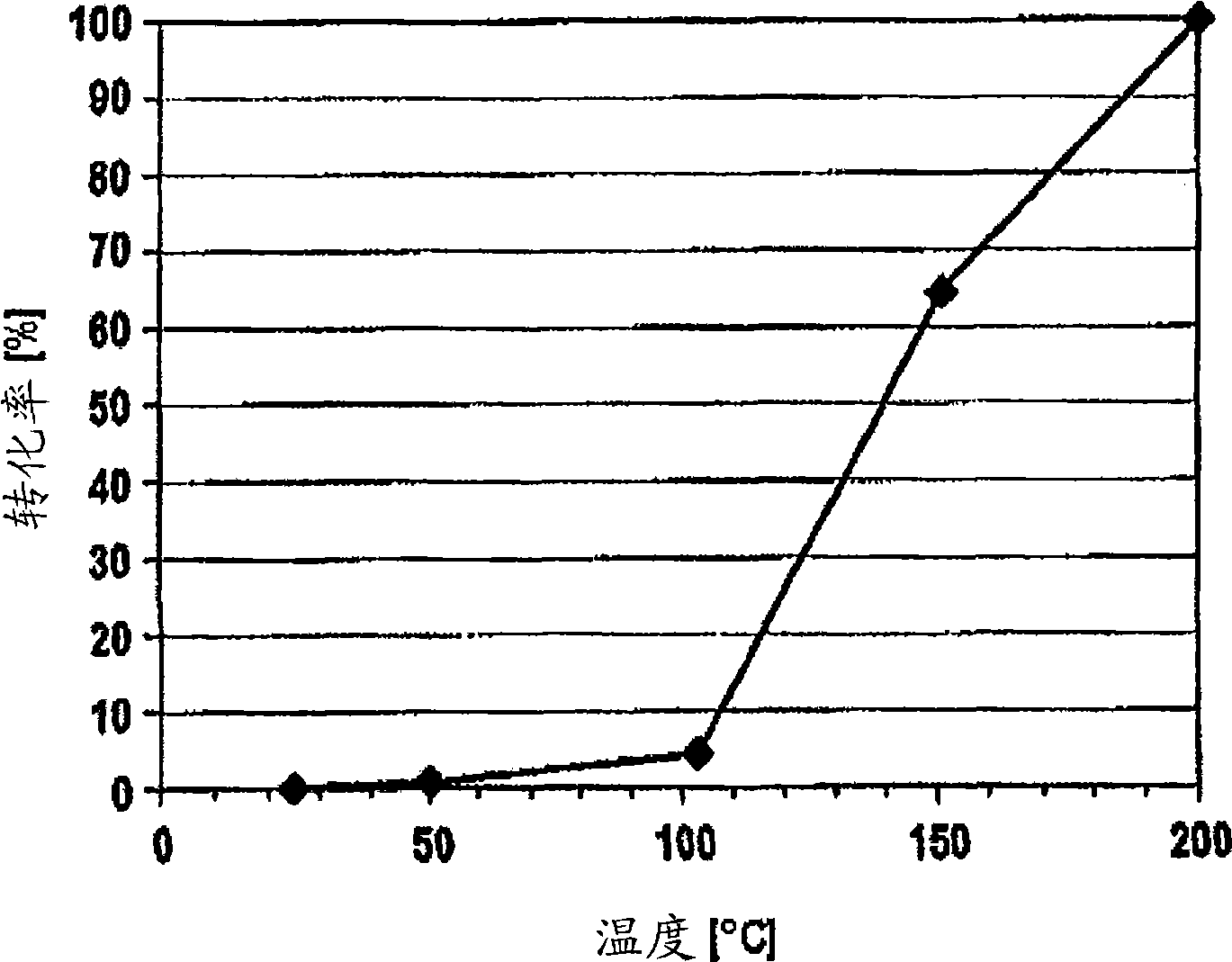
[0073] figure 1 It is shown that at a temperature of 150° C. the conversion of CO has reached about 66%. Complete conversion of CO has been achieved at a temperature of 200 °C.
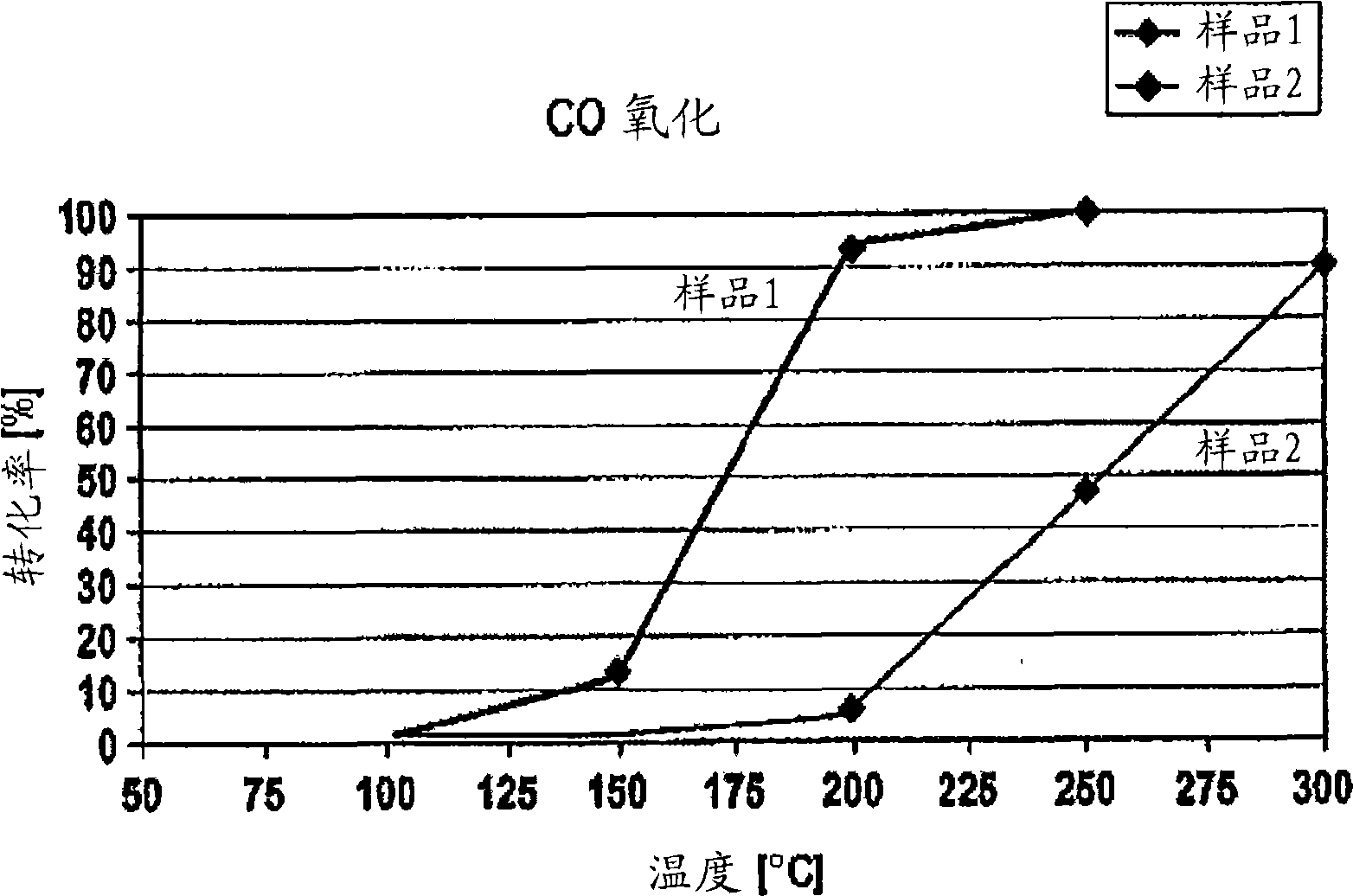
[0074] on the contrary, figure 2 shows the use of La obtained by the hydroxide method 0.5 Sr 0.5 MnO 3 CO conversion of the two samples.
[0075] As shown by the curve of sample 1, a CO conversion of about 13% was observed at a temperature of 150 °C. At a temperature of about 200°C, 90% of CO is converted, and only at a temperature of 250°C is 100% conversion obtained.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com