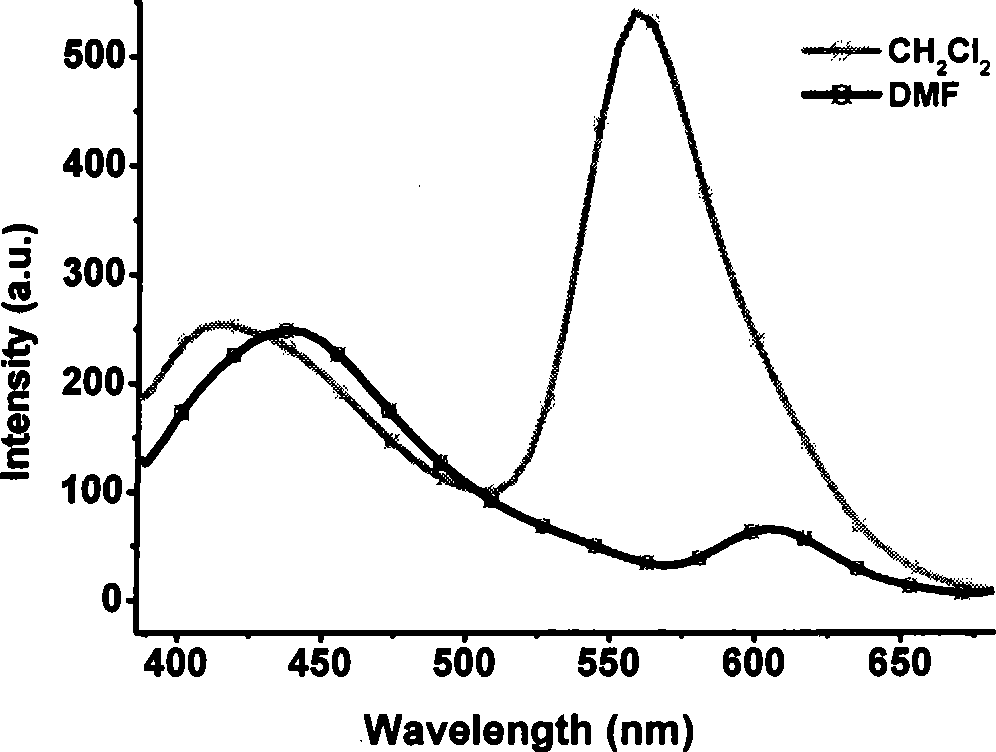
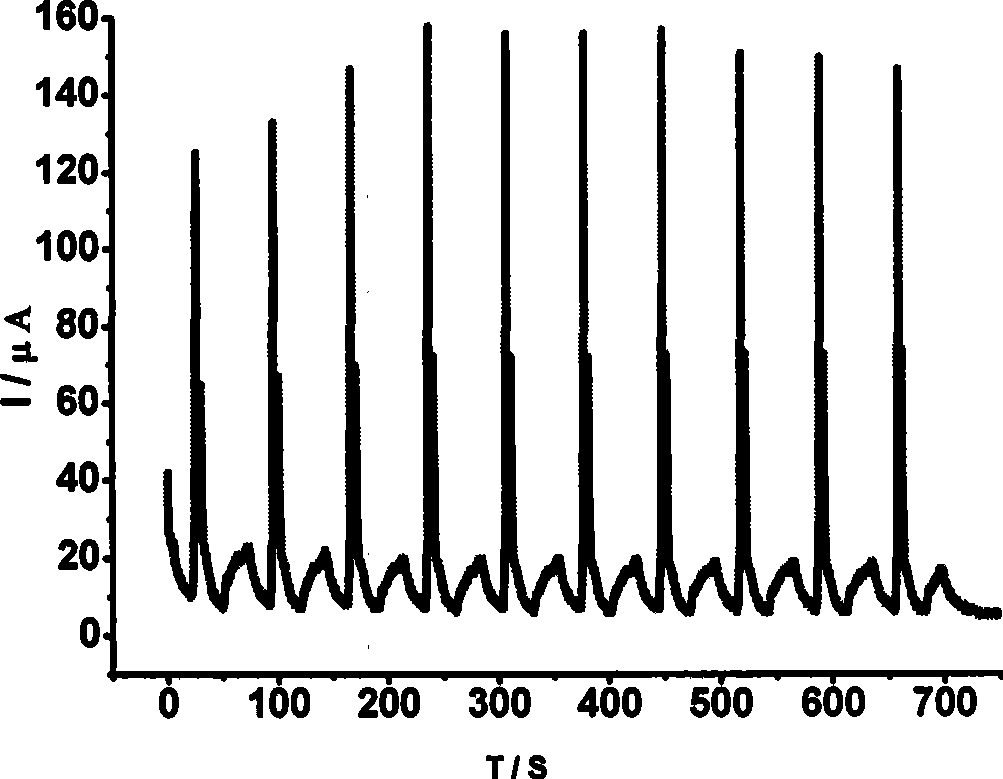
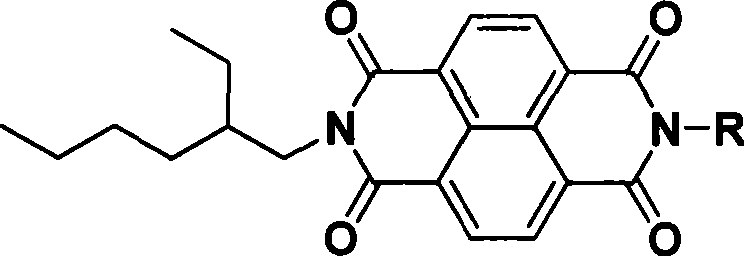
1,4,5,8-naphthalenetetracarboxylic imide derivative bifluorescent material and method of preparing the same
A technology of naphthalene tetracarboxylic acid diimide and dual fluorescence, which is applied in the fields of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problem of low quantum yield and achieve the effect of high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] Taking R as 4,6-dimethylpyrimidine as an example, non-limiting examples are described as follows:
[0045] 1. Refining of raw materials of naphthalene tetracarboxylic dianhydride: heating and dissolving the purchased industrial-grade naphthalene tetracarboxylic dianhydride raw material with KOH aqueous solution, and then recrystallizing with ethanol to obtain crystalline potassium salt of naphthalene tetracarboxylic acid, which is dissolved in an appropriate amount of water Use hydrochloric acid to adjust the pH of the solution to <3, and a large amount of white precipitates are produced. The precipitates are separated, and the precipitates are vacuum-dried to obtain relatively pure yellow naphthalene tetracarboxylic dianhydride powder.
[0046] 2. Preparation of intermediate (1):
[0047] Get 2.00g (7.46mmol) of refined naphthalene tetracarboxylic dianhydride in a three-necked flask, add 20mL DMF, 13mg (0.07mmol) zinc acetate, in N 2 When heated to ≥100°C under protec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com