Macromole polymerization inhibitor containing phenolic group and azoxynaphthalene and preparation thereof
A macromolecule and nitrogen-oxyl technology, which is applied in the field of macromolecular polymerization inhibitors containing phenolic groups and nitrogen-oxyl groups and its preparation, can solve the problem of the preparation method of macromolecular polymerization inhibitors that have not yet been seen, poor polymerization inhibition effect, inhibition Short polymerization time and other problems, to achieve a wide range of industrial application prospects, easy to operate, reduce the effect of dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
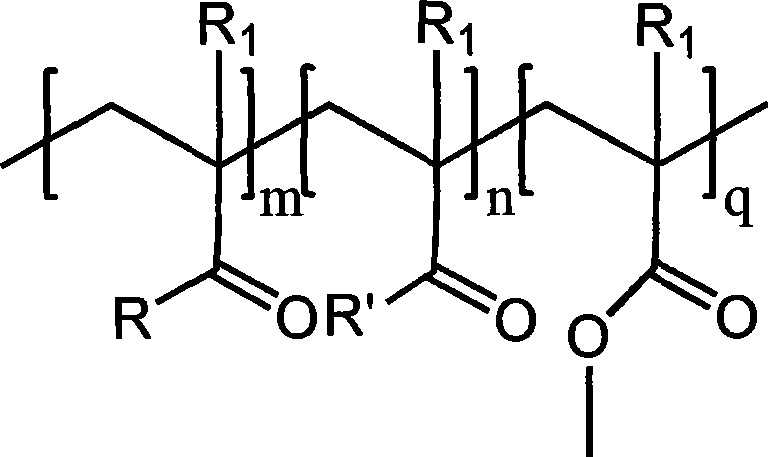
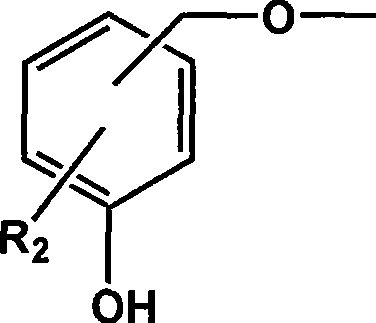
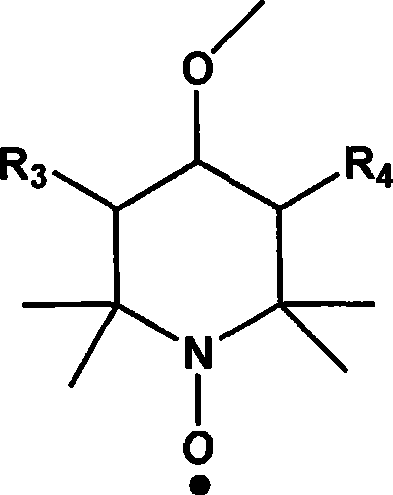
[0029] In a three-neck flask reactor equipped with a stirring device, add 200 g of polymethyl methacrylate with a molecular weight of 2000, 62 g of p-hydroxybenzyl alcohol, 2,2,6,6-tetramethyl-4-hydroxyl-piperidine nitrogen Oxygen radical 17.3g, catalyst Na 2 CO 3 5.6g (2wt%) and solvent N,N-dimethyl-formamide 800mL, stirred and dissolved, heated to 40°C, kept a vacuum of 0.09MPa, reacted until p-hydroxybenzyl alcohol and 2,2 were detected by gas chromatography, The concentration of 6,6-tetramethyl-4-hydroxy-piperidine nitroxide does not change. After the reaction mixture is cooled, it is poured into 2L of water to settle, and the product is collected by filtration and vacuum-dried to obtain a white solid product, named For MMIn 1, its yield was 88%.
[0030] In the infrared spectrum of the resulting product, 1167, 1245cm -1 The stretching vibration absorption peak of the N-O coordination bond is at 2882cm -1 The stretching vibration absorption peak of the C-N bond is at ...
Embodiment 2
[0034] In a three-neck flask reactor equipped with a stirring device, add 200 g of polymethyl methacrylate with a molecular weight of 8000, 62 g of p-hydroxybenzyl alcohol, and 2,2,6,6-tetramethyl-4-hydroxyl-piperidine nitrogen Oxygen radicals 86.5g, catalyst NaOH 5.2g (1.5wt%) and solvent N,N-dimethyl-acetamide 1000mL, stirred and dissolved, heated to 50°C, kept vacuum at 0.08MPa, reacted until detected by gas chromatography The concentration of p-hydroxybenzyl alcohol and 2,2,6,6-tetramethyl-4-hydroxyl-piperidine nitroxide radical does not change any more, after the reaction mixture is cooled, it is poured into 2L of water to settle, and the product is collected by filtration. Drying in vacuo gave a white solid product named MMIn2 with a yield of 85%.
[0035] In the infrared spectrum of the resulting product, 1167, 1245cm -1 The stretching vibration absorption peak of the N-O coordination bond is at 2882cm -1 The stretching vibration absorption peak of the C-N bond is at ...
Embodiment 3
[0039] In the existing trimethylolpropane triacrylate (TMPTA) production process, the SO 4 2- / TiO 2 / La 3+ The solid superacid catalyst is fixed in a fixed-bed reactor, and under the condition of 100±10°C, trimethylolpropane and acrylic acid are directly esterified to obtain TMPTA (original product). Then the macromolecular polymerization inhibitor MMIn1 prepared in the above-mentioned embodiment 1 of the present invention comprising phenolic groups and nitrogen-oxyl groups is loaded and fixed on the same area of the catalyst, keeping other conditions of the original process unchanged, and carrying out continuous production of TMPTA, and the resulting product is TMPTA product 1; reduce the consumption of small molecule polymerization inhibitor to 1 / 2 of the original process, keep other production conditions unchanged, and the resulting product is TMPTA product 2. A comparison of some of their physical properties with the original product is listed in Table 1 below.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com