A cellular therapy for ocular degeneration
A cell and stem cell technology, applied in the field of cell-based therapy or regenerative therapy, which can solve the problem of low expansion ability of adult stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
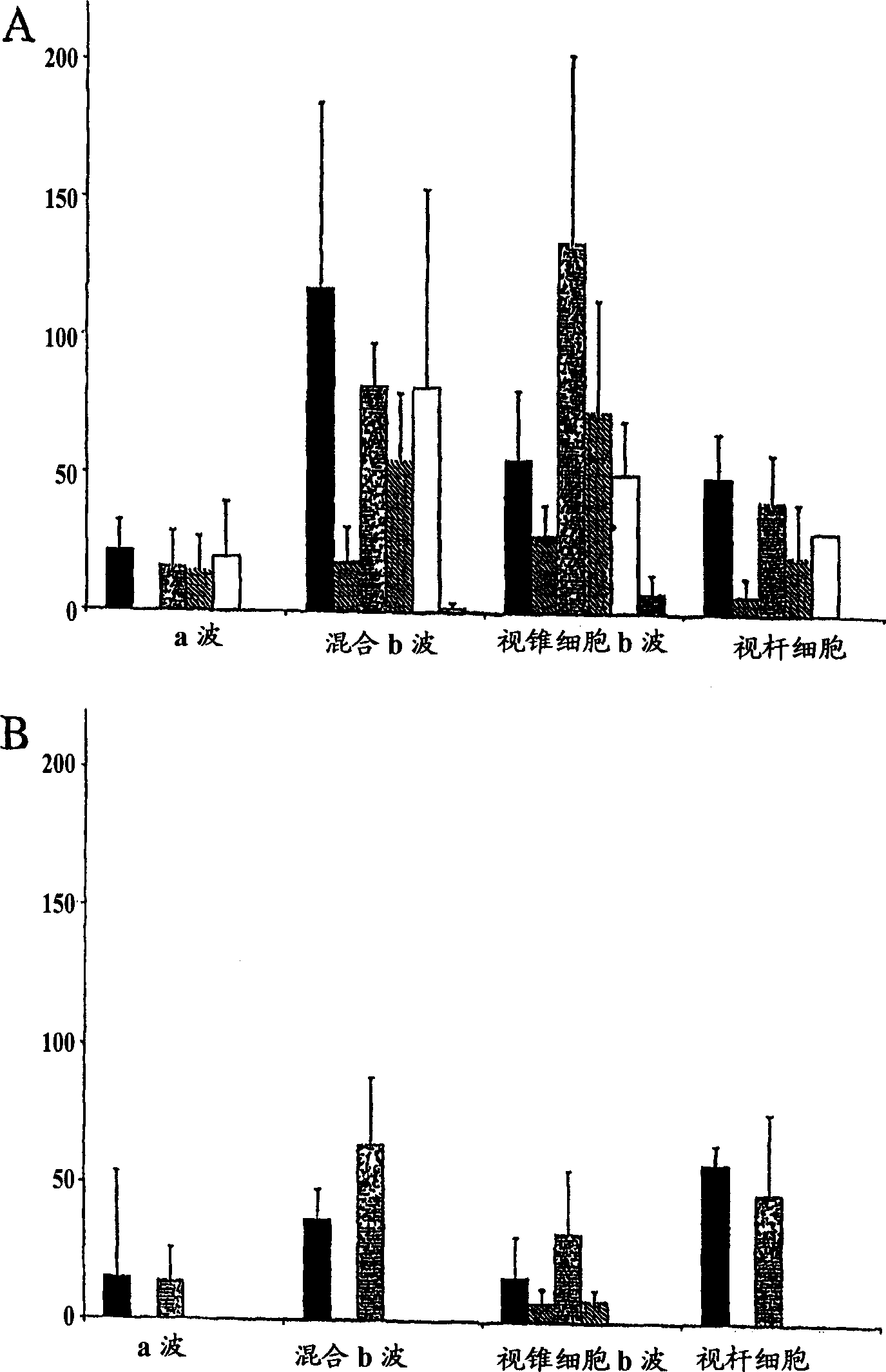
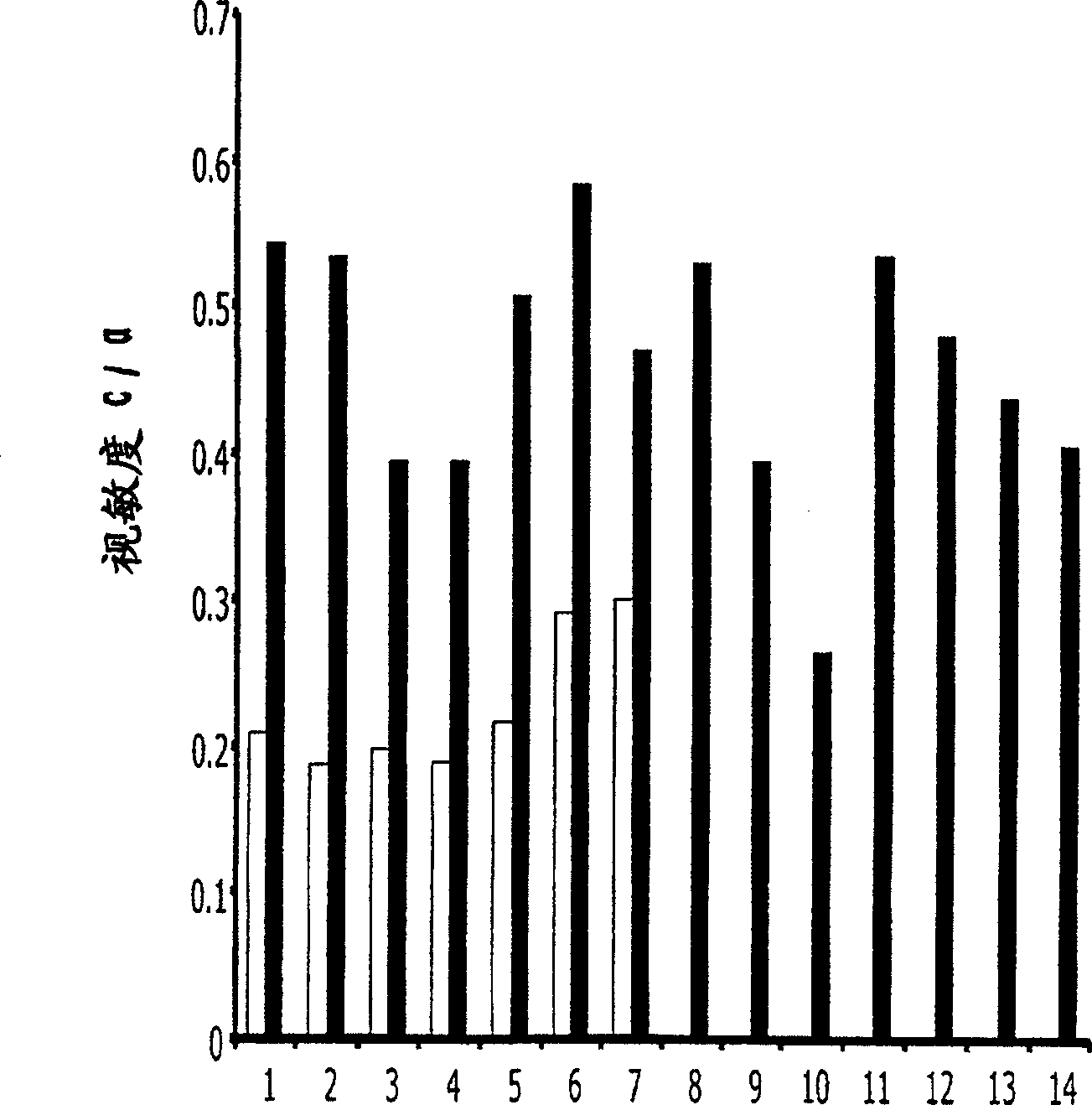
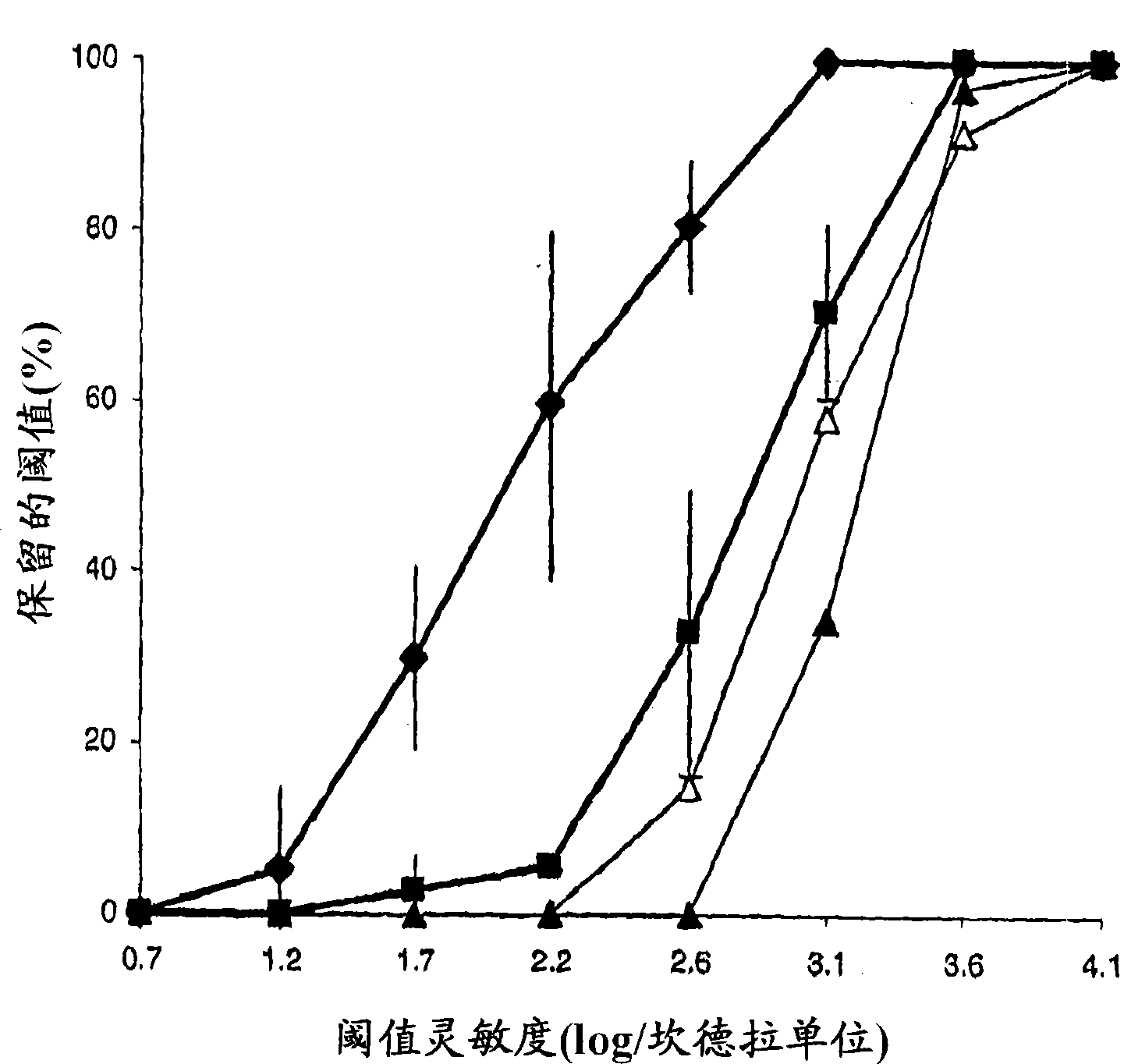
[0097] Use of mesenchymal stem cells in the treatment of retinitis pigmentosa
[0098] Genetically engineered Royal College of Surgeon (RCS) rats are predisposed to experience significant visual loss caused by fundamental dysfunction of retinal pigment epithelial (RPE) cells. Using this model, the efficacy of subretinal transplantation of mesenchymal stem cell populations to protect photoreceptors was determined in RCS rats. In this degenerative model, rod function is lost within 30-60 days of life. Cone function is usually lost within 3 months.
[0099] Preparation of MSCs for transplantation
[0100] Adult MSC cultures (cat# PT-2501, Cambrex) were expanded in vitro for a total of 6 passages. All cells at 5,000 cells / cm 2 First inoculated in uncoated T75 flasks containing mesenchymal stem cell growth medium supplemented according to the manufacturer's (Cambrex) instructions. For subsequent passages, all cells were processed as follows. After trypsin hydrolysis, viable c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com