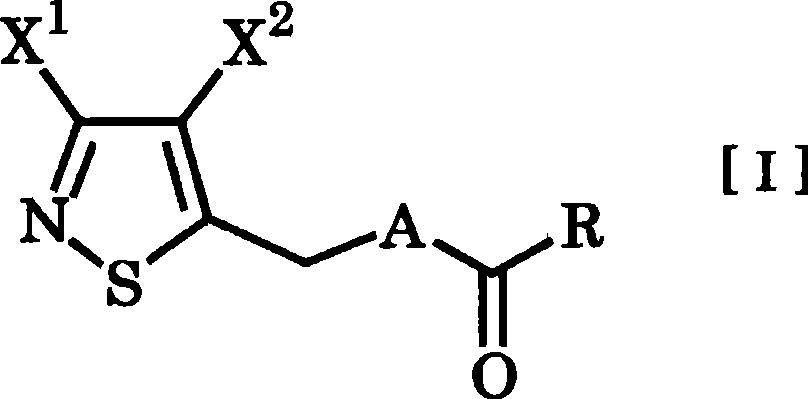
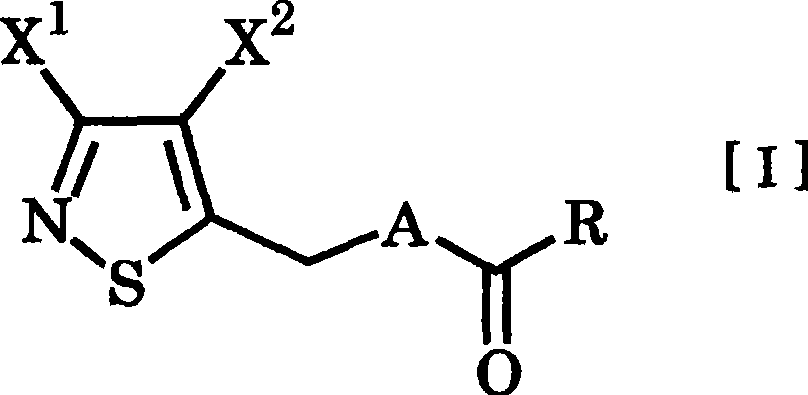
3,4-dihalogenoisothiazole derivative and agent for controlling agricultural or horticultural plant disease
一种异噻唑、衍生物的技术,应用在杀生物剂、植物生长调节剂、杀生剂等方向,能够解决没有公开等问题,达到高防除效果的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Production of (3,4-dichloroisothiazol-5-yl)methylbenzoate (Compound No. II-1 of the present invention)
[0160] Add 8 ml of oxalyl chloride and a catalytic amount of N,N-dimethylformamide (DMF) to 4.0 g (20.3 mmol) of 3,4-dichloroisothiazole-5-carboxylic acid, and stir at 50° C. for 30 minutes. The reaction mixture was concentrated under reduced pressure to obtain 3,4-dichloroisothiazole-5-carbonyl chloride.
[0161] Suspend 1.9g (50.5mmol) of sodium borohydride in 40ml of water, and drop THF (4ml ) solution. Stir at 15°C for 30 minutes, then add aqueous citric acid to make the solution weakly acidic, and extract with ethyl acetate. The organic layer was washed with water, dried over anhydrous magnesium sulfate, and concentrated. The obtained crystals were washed with hexane to obtain 3.0 g (yield 81%) of colorless crystals of (3,4-dichloroisothiazol-5-yl)methanol (melting point 94-95°C).
[0162] 1 H-NMR data (CDCl 3 / TMSδ (ppm)): 2.28 (1H, bs), 4.96 (2H, s) ppm
...
Embodiment 2
[0166] Production of (3,4-dichloroisothiazol-5-yl)methylthioacetate (Compound No. I-28 of the present invention)
[0167] To a solution of 2.85 g (10.9 mmol) of triphenylphosphine in THF 50 ml, 2.2 g (10.9 mmol) of diisopropyl azodicarboxylate was added under ice-cooling, followed by stirring for 30 minutes. A THF 5 ml solution of 1.0 g (5.43 mmol) of (3,4-dichloroisothiazol-5-yl)methanol and 0.83 g (10.9 mmol) of thioacetic acid was added to this solution, and stirred at room temperature for 5 hours. After completion of the reaction, ethyl acetate was added to the reaction mixture to wash with water, dried over anhydrous magnesium sulfate, and concentrated. The residue was purified by silica gel column chromatography to obtain 0.42 g (yield 32%) of (3,4-dichloroisothiazol-5-yl) methylthioacetate as a light red oily substance (refractive index 1.5855).
[0168] 1 H-NMR data (CDCl 3 / TMS δ (ppm)): 2.41 (3H, s), 4.24 (2H, s)
[0169] The physical property values of the co...
Embodiment 3
[0176] [embodiment 3] powder
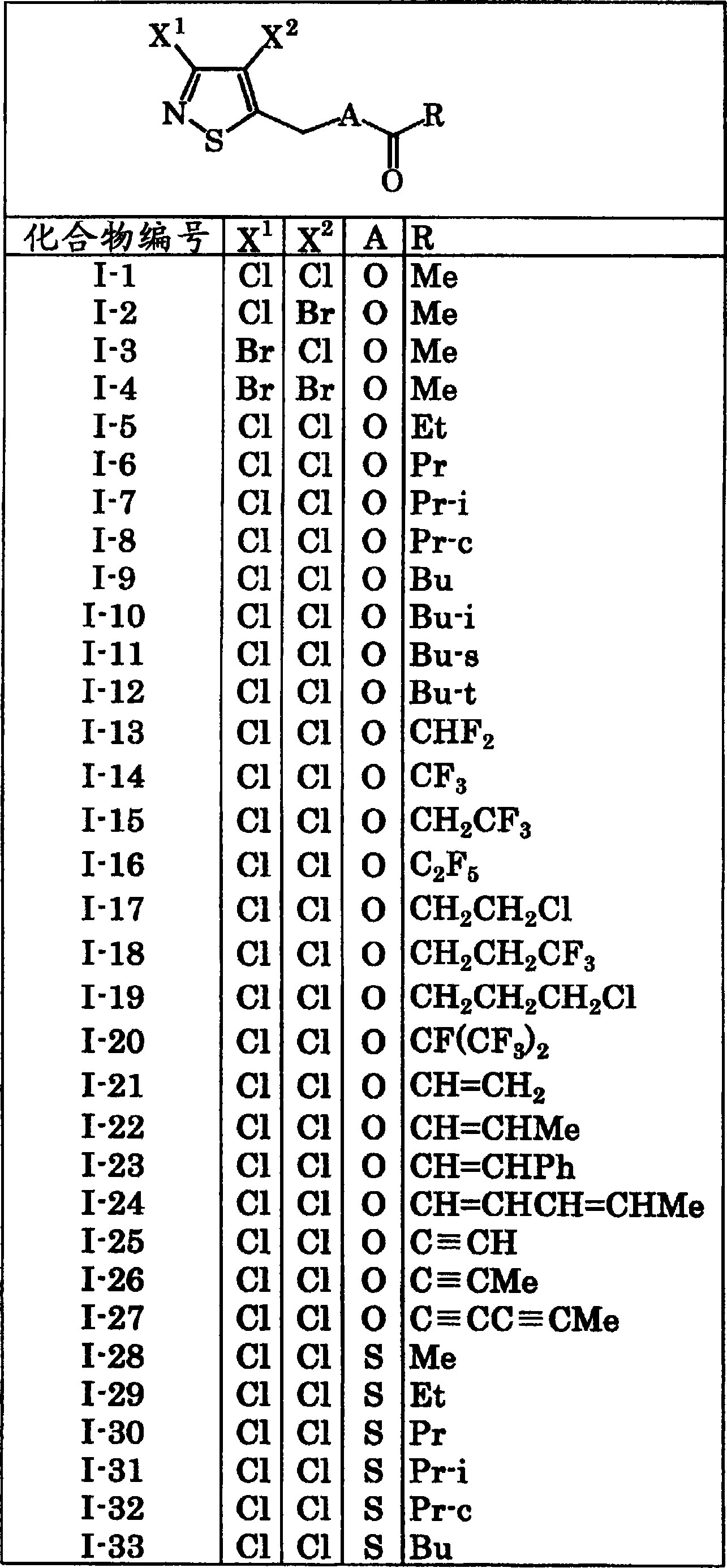
[0177] 2 parts of the compound of compound number I-1
[0178] Diatomaceous earth 5 parts
[0179] Clay 93 parts
[0180] The above ingredients are uniformly mixed and pulverized to make a powder. In addition, powders can be obtained in the same manner by using the compounds described in Tables 1 to 10 instead of Compound No. I-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com