High dose oral pharmaceutical compositions of artemether and lumefantrine
A technology of artemether and composition, applied in the field of high-dose oral pharmaceutical composition, can solve the problems of patient non-compliance, low reliability, mental confusion and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
(80+480mg) artemether 80.0 benzefantrine 480.0 Microcrystalline Cellulose (MCC 112) 250.0 Hydroxypropylmethylcellulose 20.0 Croscarmellose Sodium 120.0 Colloidal silica 20.0 Magnesium stearate 30.0 Tablet weight 1000.0
[0054] 1. Artemether and lumefantrine are sieved through a specific sieve together with microcrystalline cellulose, hydroxypropyl methylcellulose and croscarmellose sodium, and blended in a double-cone mixer to obtain a blend compound.
[0055] 2. Blend the blend of step 1 with the sieved colloidal silicon dioxide and magnesium stearate to obtain the final blend.
[0056] 3. Compress the final blend of step 2 using approved processing methods to obtain tablets.
Embodiment 2-4
80+480mg Intragranular artemether 40.0 60.0 80.0 benzefantrine 240.0 360.0 480.0 microcrystalline cellulose 42.6 63.9 85.2 Croscarmellose Sodium 56.0 84.0 112.0 Hydroxypropylmethylcellulose 8.0 12.0 16.0 Colloidal silica 10.0 15.0 20.0
[0059] Extragranular microcrystalline cellulose 20.0 30.0 40.0 Polysorbate 80 1.0 1.5 2.0 microcrystalline cellulose 56.4 84.6 112.8 Colloidal silica 6.0 9.0 12.0 Magnesium stearate 20.0 30.0 40.0 total 500.0 750.0 1000.0
[0060] 1. Artemether and lumefantrine are sieved through a specific sieve and mixed geometrically to obtain a mixture.
[0061] 2. Add microcrystalline cellulose, hydroxypropylmethylcellulose, croscarmellose sodium and colloidal silicon dioxide to the mixture obtained in step 1 to obtain a blend.
[0062] 3. Compress the blend obtained in step 2 to obtain a pre-compressed tablet, det...
Embodiment 2
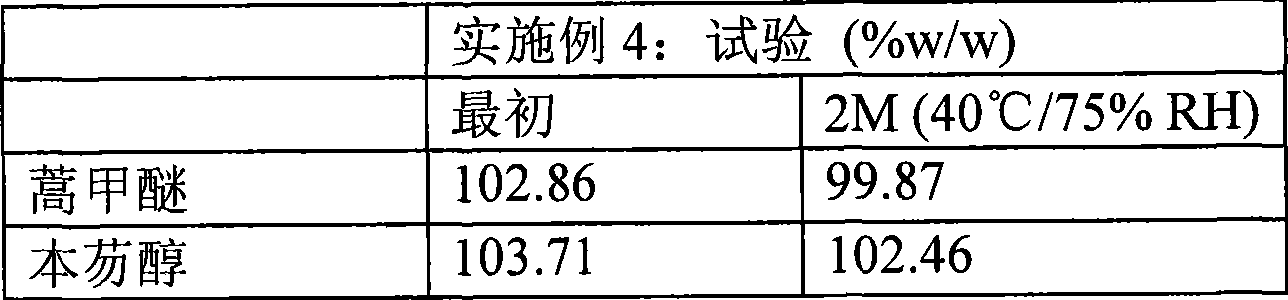
[0078] The experimental value of embodiment 2
[0079]
Example 2
Test (%w / w) artemether 103.93 benzefantrine 100.07
[0080] Table 4
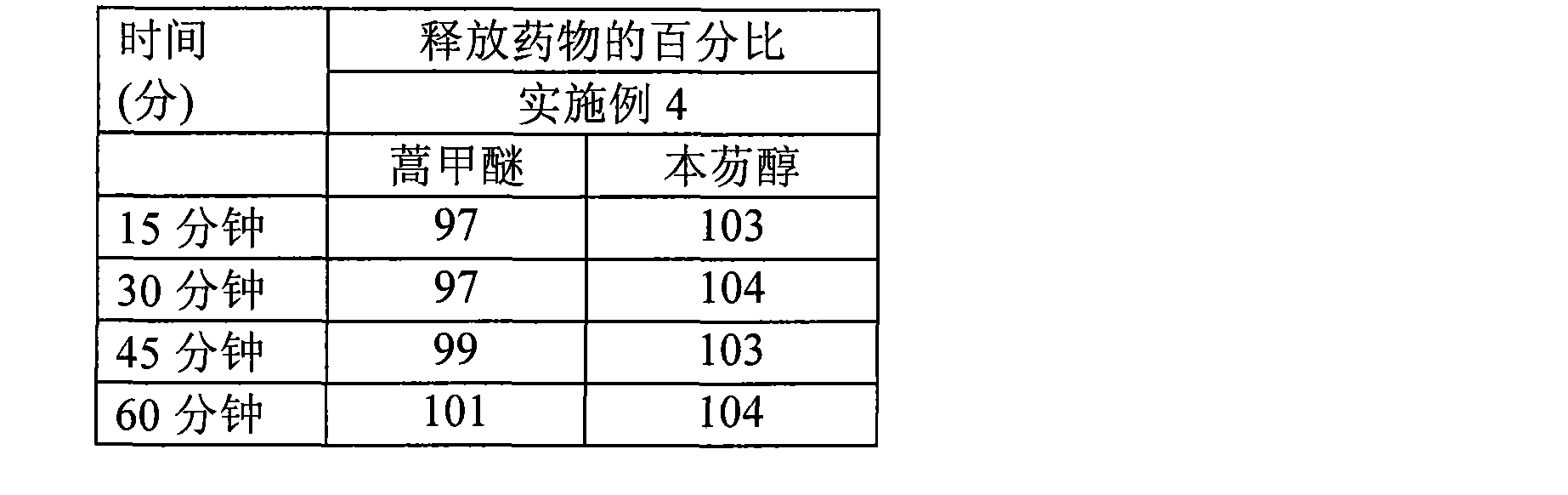
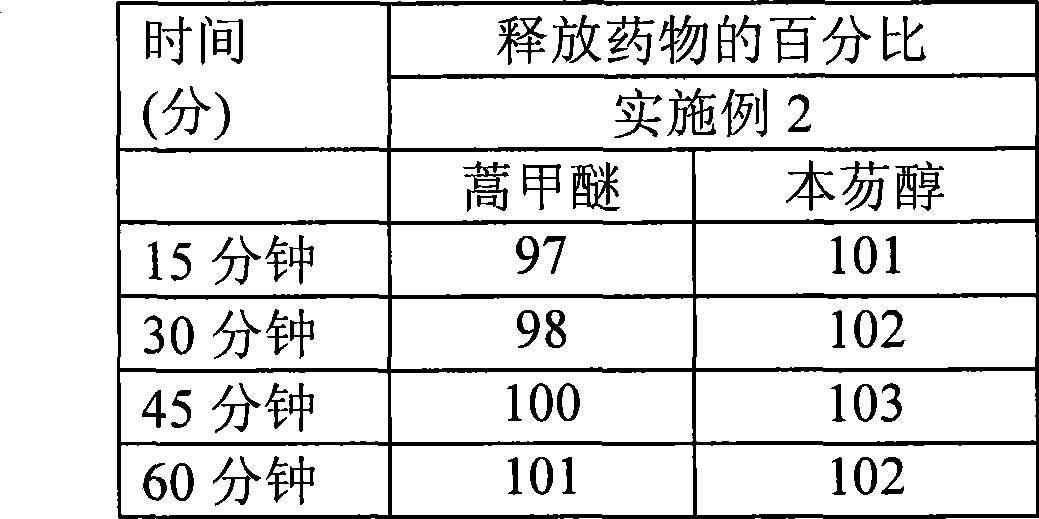
[0081] The dissolution curve of embodiment 2
[0082] Artemether: Dissolution curve obtained by USPI type II method at 100 rpm in 1800 ml pH 7.0 phosphate buffer + 3.0% sodium lauryl sulfate.
[0083] Benefantrine: Dissolution curve in 1800 ml 0.1N HCl + 3.0% benzalkonium chloride using USP Type II method at 75 rpm.
[0084]
[0085] While specific high dose oral pharmaceutical compositions have been described above, it will be apparent that various modifications and combinations can be made to the compositions detailed herein without departing from the spirit and scope of the invention. For example, other exemplary tablet formulations were contemplated for preparing high dose oral pharmaceutical compositions of artemether and lumefantrine, as described in Examples 5-12.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com