Catalyst for reducing nitrogen oxides and process for reducing nitrogen oxides
A technology for purifying catalysts and nitrogen oxides, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Durability iron silicate and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Dissolve aluminum nitrate nonahydrate 18.79g, iron nitrate nonahydrate 4.63g in 257g tetraethylammonium hydroxide 35% aqueous solution (hereinafter referred to as "TEAOH"), then add tetraethyl orthosilicate (TEOS) 209g, Thoroughly stir and mix, carry out hydrolysis at room temperature, and evaporate the ethanol generated. Next, the necessary amount of water is evaporated. 20.88 g of 48% hydrofluoric acid was added thereto, and after mixing with a mortar, the reaction mixture was added to a stainless steel autoclave, and heated at 150° C. for 240 hours to crystallize. The composition of the reaction mixture is 40SiO 2 :Al 2 o 3 :0.23Fe 2 o 3 :20HF:24.4TEAOH:300H 2 O. The crystallized syrupy mixture was white. This mixture was subjected to solid-liquid separation, washed with pure water, and dried at 110°C.
[0161] The dry powder was calcined at 600°C for 2 hours under air circulation. As a result of X-ray diffraction measurement of the obtained β-type iron sil...
Embodiment 2
[0163] In addition to changing the composition ratio of the crystallized reaction mixture to 70SiO 2 :Al 2 o 3 :0.47Fe 2 o 3 :35HF:42TEAOH:490H 2Except for O, a reaction mixture was prepared by the same procedure as in Example 1. This reaction mixture was added to a stainless steel autoclave, and crystallized by heating at 150°C for 160 hours. The crystallized syrupy mixture was white. This mixture was subjected to solid-liquid separation, washed with a sufficient amount of pure water, and dried at 110°C. The dry powder was calcined at 600°C for 2 hours under air circulation.
[0164] From the X-ray diffraction measurement results of the obtained β-type iron silicate, it was found that the X-ray diffraction pattern shown in Table 1 was obtained.
Embodiment 3
[0166] In addition to changing the composition ratio of the crystallized reaction mixture to 70SiO 2 :Al 2 o 3 :Fe 2 o 3 :35HF:42TEAOH:490H 2 Except for O, a reaction mixture was prepared in the same manner as in Example 1. This reaction mixture was added to a stainless steel autoclave, and heated at 150° C. for 240 hours to crystallize. The crystallized syrupy mixture was white. This mixture was subjected to solid-liquid separation, washed with a sufficient amount of pure water, and dried at 110°C. The dry powder was calcined at 600° C. for 2 hours under air circulation.
[0167] As a result of the X-ray diffraction measurement of the obtained β-type iron silicate, the X-ray diffraction pattern shown in Table 1 was obtained.
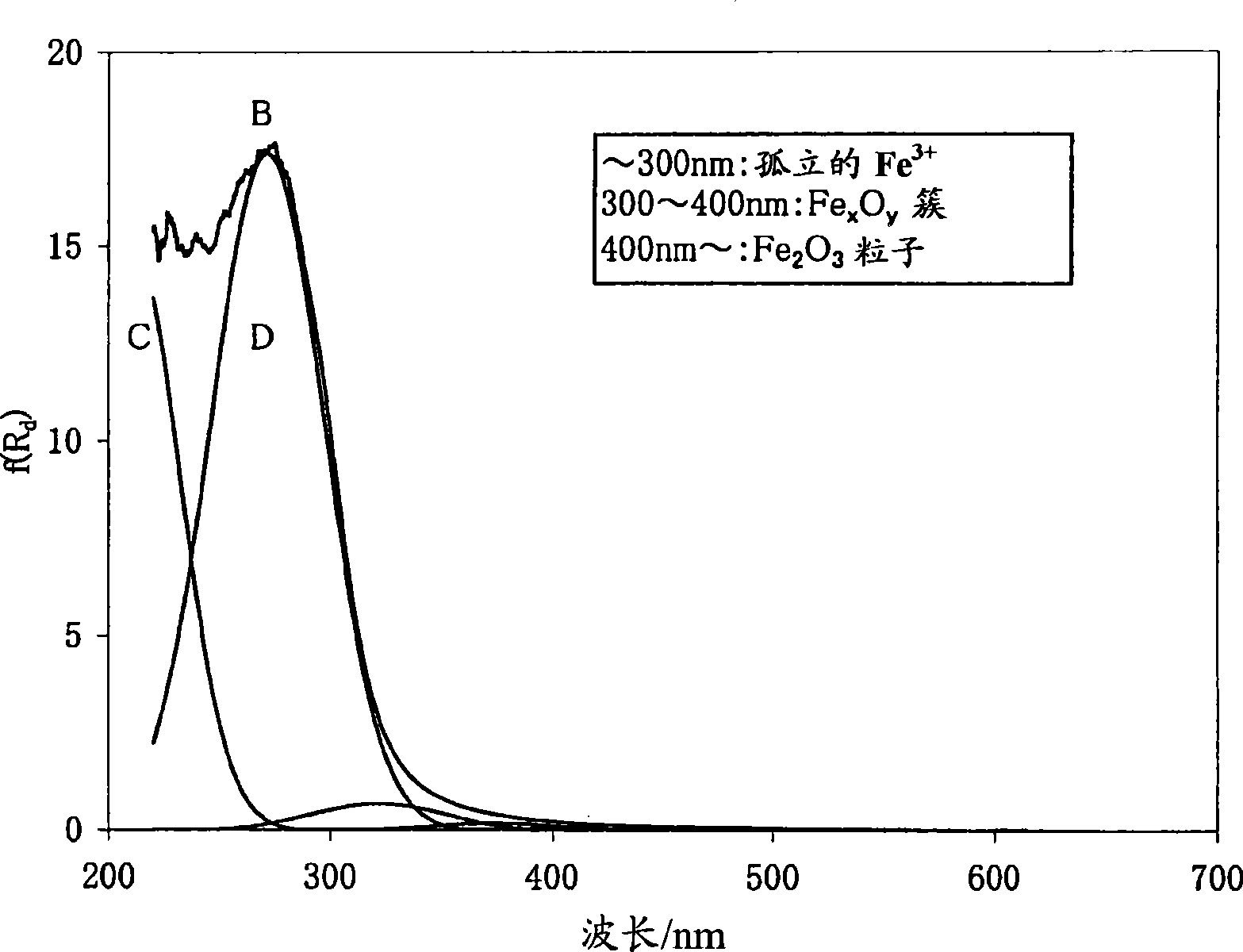
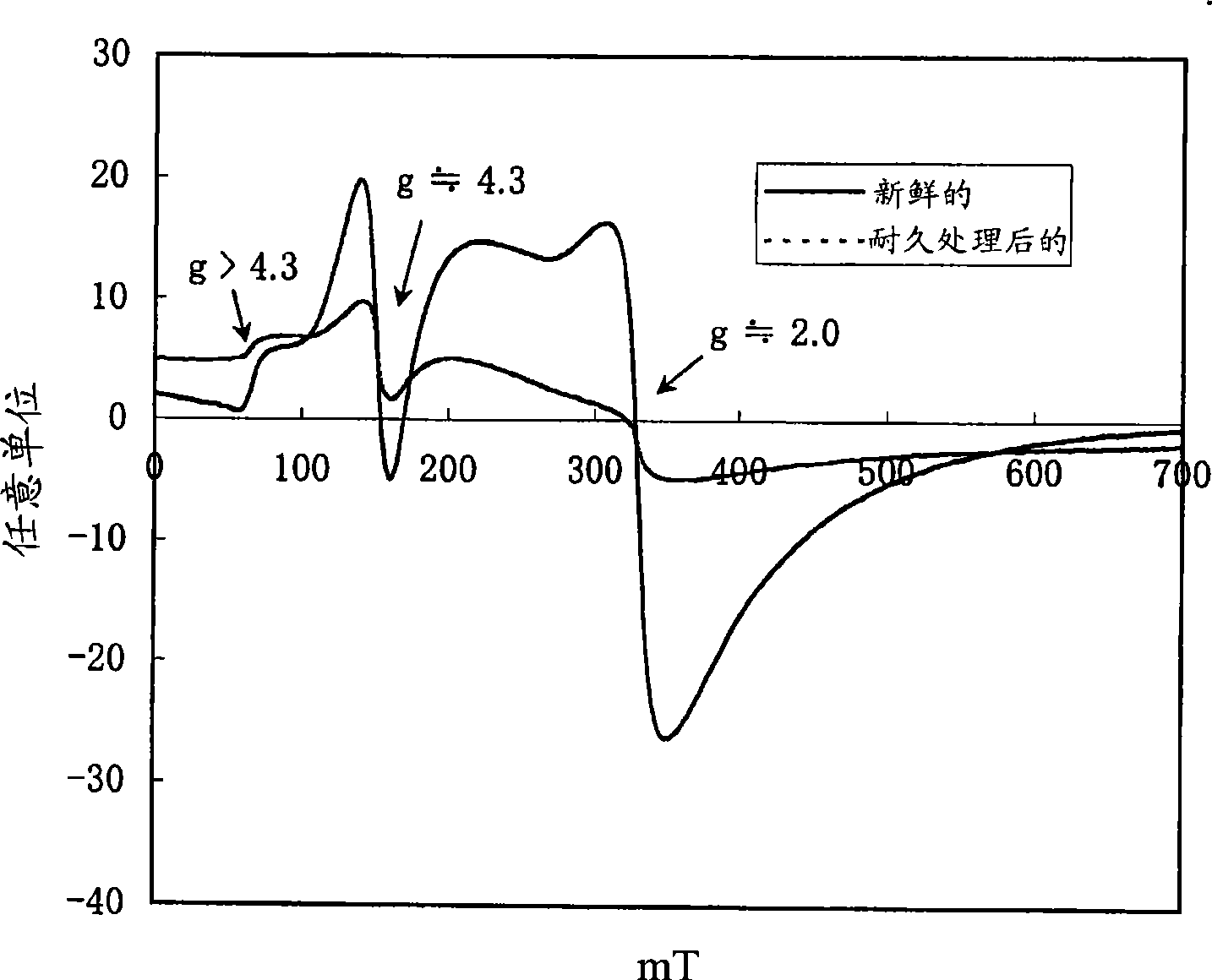
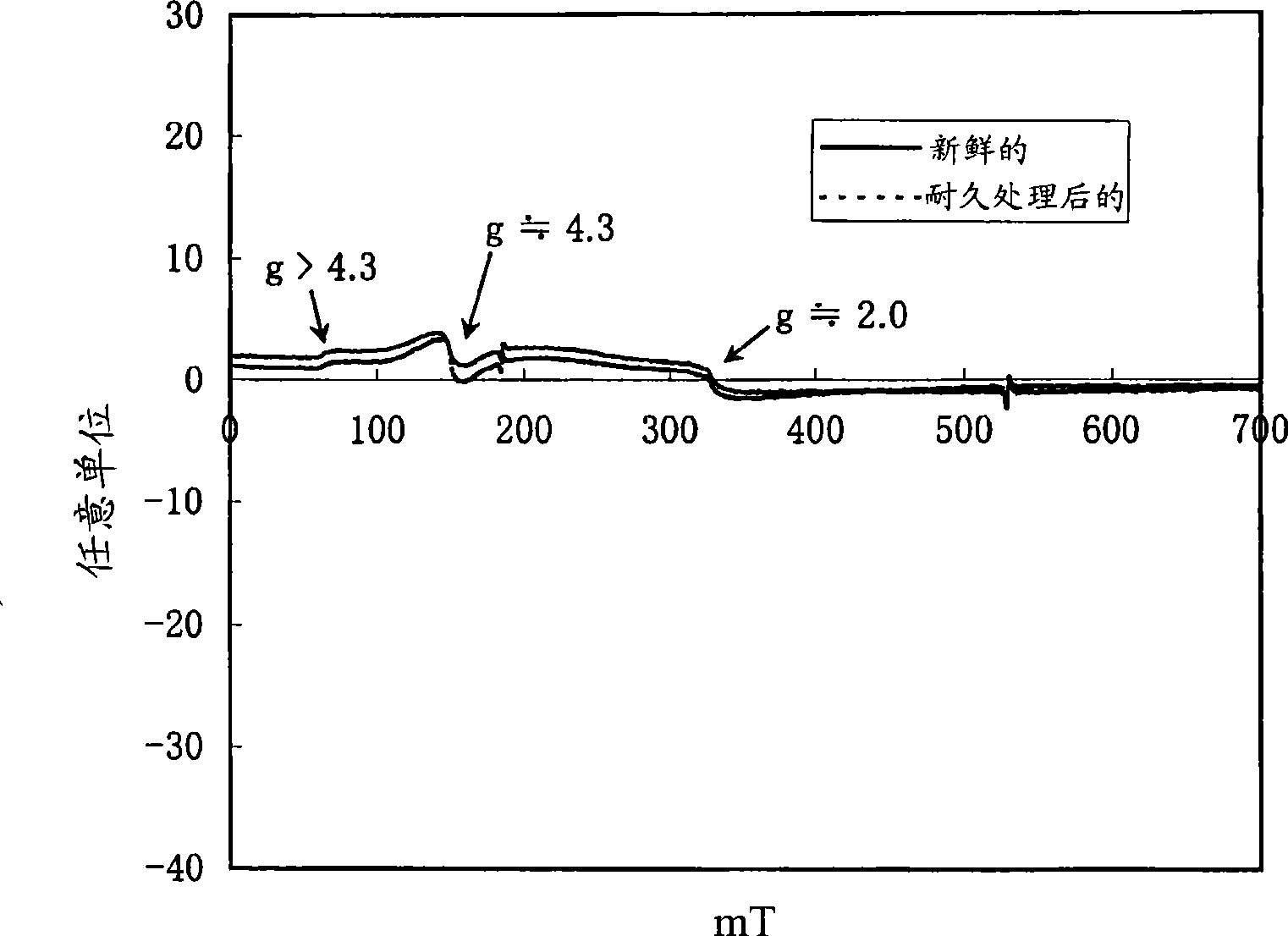
[0168] figure 1 The ultraviolet-visible absorption spectrum of β-type iron silicate (before durability treatment) is shown. figure 2 An example of electron spin resonance spectra of β-type iron silicate 1 before and after durability treatment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystal size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com