Ortho-metallated hafnium complexes of imidazole ligands
A technology of metal complexes and ligands, applied in the field of olefin polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0201] The following specific embodiments of the invention and combinations thereof are particularly desirable and are therefore described to provide a detailed disclosure of the appended claims.
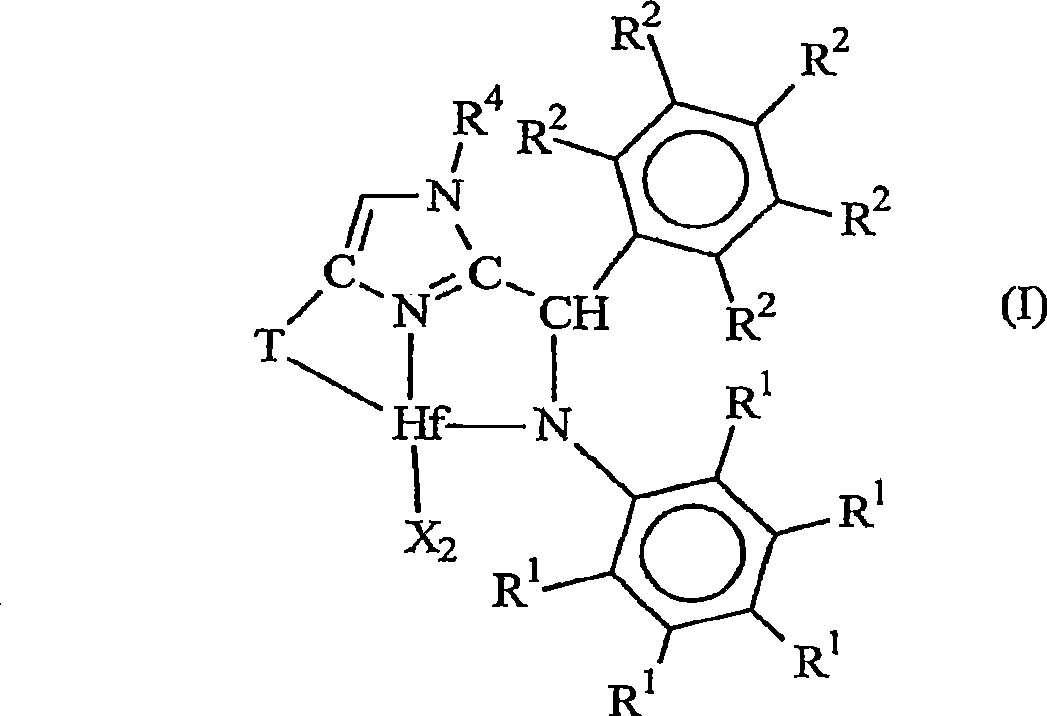
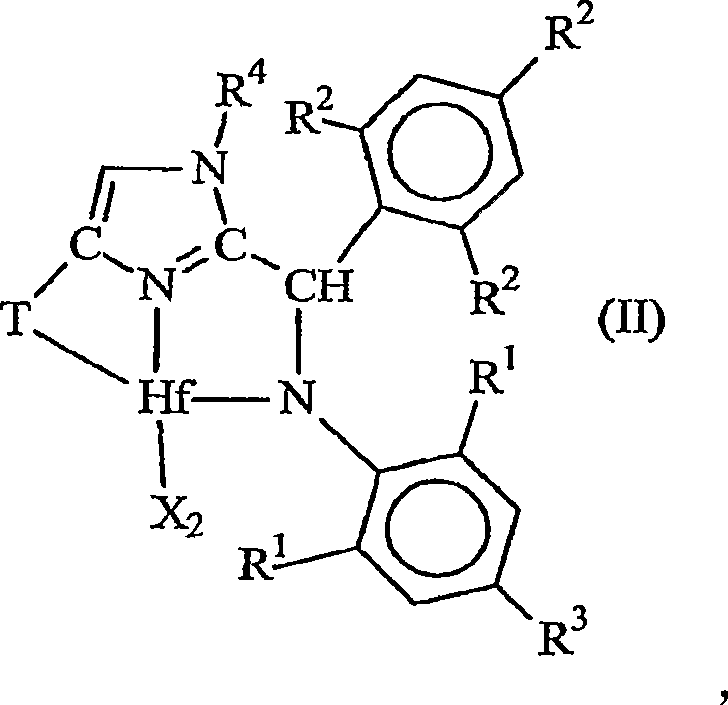
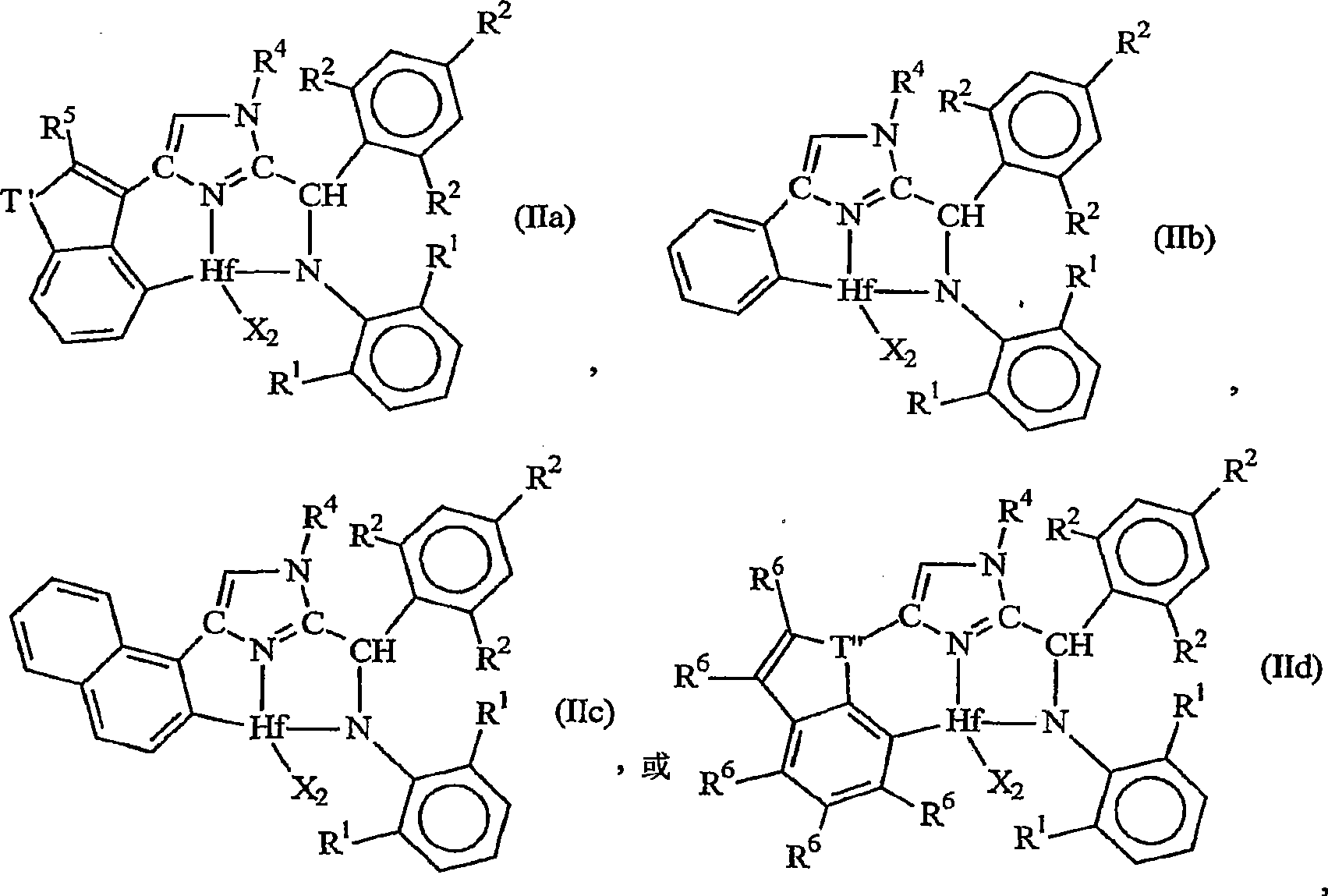
[0202] 1. A metal complex corresponding to the following general formula:
[0203]
[0204] Wherein, X is an anionic ligand independently, or two X groups together form a dianionic ligand group or a neutral diene, preferably X is C 1-20 Hydrocarbyl, trihydrocarbylsilyl or trihydrocarbylsilylhydrocarbyl groups;
[0205] T is a cycloaliphatic or aromatic group containing one or more rings;
[0206] R 1 are independently hydrogen, halogen, or monovalent, polyatomic anionic ligands, or two or more R 1 The groups bond together thus forming a polyvalent fused ring system;
[0207] R 2 are independently hydrogen, halogen, or monovalent, polyatomic anionic ligands, or two or more R 2 The groups are joined together to form a polyvalent fused ring system; and
[0208] R 4 is hydrog...
Embodiment 1
[0257] Example 1[N-[2,6-bis(1-methylethyl)phenyl]-α-[2,4,6-three(1-methylethyl)phenyl]-5-(2 -Ethylbenzofuran-3,4-diyl-κ-C 4 )-2-(N'-methyl)imidazol-2-yl)methanamine (2-)-kN 1 , κN 2 ] Di(methyl)hafnium
[0258]
[0259] (a) In a 250 mL flask with magnetic stirring, 100 mL of diethyl ether and 2-ethylbenzofuran (20.0 g, 137 mmol) were added. The reaction flask was then cooled to 0 °C. Bromine (8.40 mL, 164 mmol) was then added to the dropping funnel containing 50 mL of ethyl acetate. The mixture was added dropwise into the reactor maintaining a temperature of 0 °C. An additional 20 mL of ethyl acetate was used to rinse the dropping funnel. The resulting mixture was stirred for 2 hours keeping the temperature at 0 °C. The reaction was quenched with 50 mL of water. The contents of the reactor were then transferred to a 1 L separatory funnel and rinsed with 2 x 50 mL of water. The organic layers were combined and washed with 200 mL of saturated sodium thiosulfate solut...
Embodiment 2
[0273] Example 2[N-[2,6-bis(1-methylethyl)phenyl]-α-[2,4,6-three(1-methylethyl)phenyl]-5-(2 -Ethylbenzofuran-3,4-diyl-κ-C 4 )-2-(N′-methyl)imidazol-2-yl)methanamine (2-)-κN 1 , κN 2 ] Di(n-butyl) hafnium
[0274]
[0275] (a) 2-(1) N-methylimidazole methylamine, N-[2,6-bis(1-isopropyl)phenyl]-α-[2,4,6-(triisopropyl )Phenyl]4-3(2-ethylbenzofuran) (Example 1(f), 0.81 mmol dissolved in 20 mL toluene) was charged into a glass flask. To this solution was added 0.81 mmol of n-butyllithium (2.5M solution in hexane) via syringe. The solution was stirred for 30 minutes and the toluene was removed using a vacuum system attached to the dry box. Hexane was added and removed in vacuo, added again, and the resulting slurry was filtered to form the lithium salt as a white solid (0.20 g, 0.32 mmol; 40%). A glass jar was then filled with the white solid dissolved in 30 mL of toluene. To this solution was added 0.32 mmol of solid HfCl 4 . The flask was capped using an air-cooled ref...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com