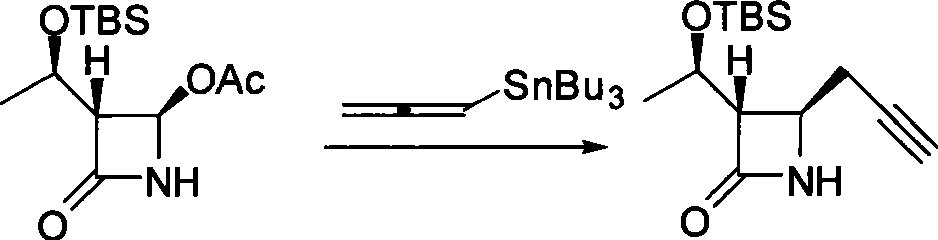
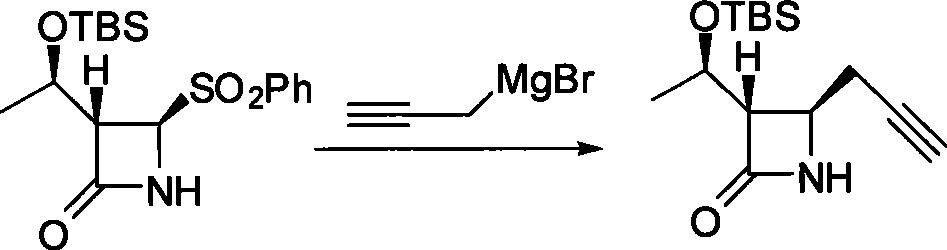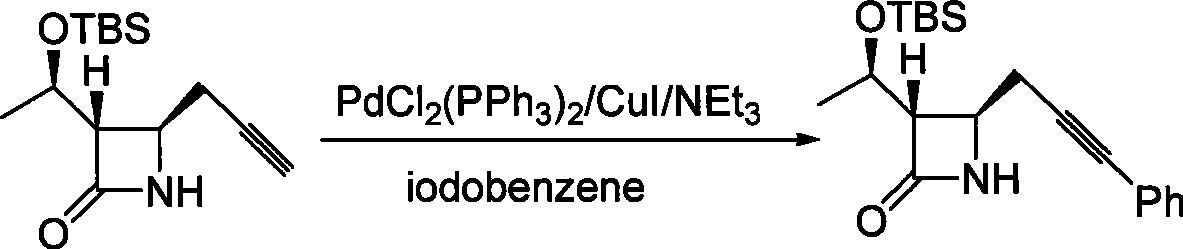Method for preparing 4-propargyl nitrogen heterocyclic butyl-2-one and 4-allenyl nitrogen heterocyclic butyl-2-one
A technology of azetidine and propargyl nitrogen, applied in the field of preparing 4-propargyl azetidin-2-one and 4-alkenyl azetidin-2-one, which can solve unfavorable industrialization , complex operation and other issues, to achieve the effect of simple route, cheap and easy-to-obtain raw materials, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 (3S, 4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-(2-propargyl)azetidin-2-one and (3S,4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-(1,2-alkenyl)azetidin-2-one Synthesis
[0029] (2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl-4-acetylazetidin-2-one (115mg, 0.4mmol), zinc Powder (52mg, 0.8mmol) and propargyl bromide (1mmol) were added to 3mL tetrahydrofuran, heated, reacted for 6 hours, cooled to room temperature, quenched by adding water (8mL), extracted with ethyl acetate (10mL×2 times). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure. Post-treatment gave (3S, 4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-( 2-propargyl)azetidin-2-one, 79% yield. and (3S,4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl) -4-(1,2-Allenyl)azetidin-2-one, 2% yield.
Embodiment 2
[0030] Example 2 (3S, 4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-(2-propargyl)azetidin-2-one and (3S,4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-(1,2-alkenyl)azetidin-2-one Synthesis
[0031] (2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl-4-acetylazetidin-2-one (115mg, 0.4mmol), zinc Powder (52mg, 0.8mmol) and propargyl chloride (1mmol) were added to 3mL diethyl ether, reacted at 0°C for 24 hours, raised to room temperature, quenched by adding water (10mL), and extracted with dichloromethane (10mL×2 times). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure. Post-treatment gave (3S, 4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-( 2-propargyl)azetidin-2-one, 54% yield. and (3S,4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl) -4-(1,2-Allenyl)azetidin-2-one, 2% yield.
Embodiment 3
[0032] Example 3 (3S, 4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-(2-propargyl)azetidin-2-one and (3S,4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-(1,2-alkenyl)azetidin-2-one Synthesis
[0033](2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl-4-benzoylazetidin-2-one (145mg, 0.4mmol), Ketone powder (51.2 mg, 0.8 mmol) and propargyl fluoride (1 mmol) were added to 3 mL of toluene, heated to reflux, reacted for 6 hours, cooled to room temperature, quenched by adding water (8 mL), extracted with ether (10 mL×2 times ). The combined organic phases were dried over anhydrous sodium sulfate and concentrated under reduced pressure. Post-processing gave (3S, 4R)-3-((R)-1-(tert-butyldimethylsilyloxy) ethyl)-4 -(2-propargyl)azetidin-2-one, yield 8%. and (3S,4R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl yl)-4-(1,2-alkenyl)azetidin-2-one, 2% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com