Tuberculosis gene vaccine assembled by chitosan delivery system and preparation method and use thereof
A technology of chitosan and gene vaccine, applied in the fields of application, gene therapy, genetic engineering, etc., can solve the problems of poor prevention and treatment of tuberculosis and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
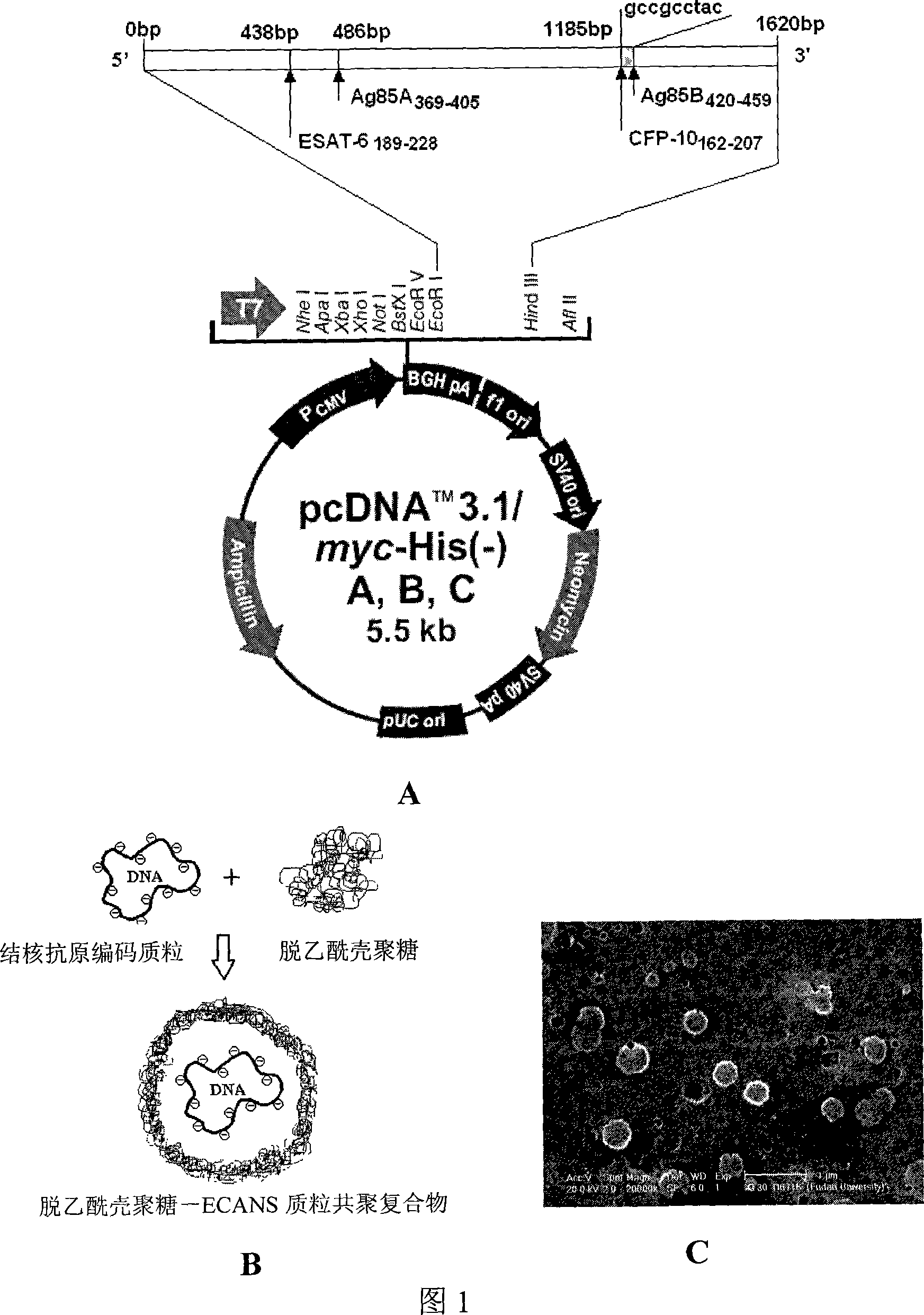
[0146] Embodiment 1: Construction of pcDNA3.1-ECANS tuberculosis gene vaccine
[0147] 1. Prediction of target T cell epitopes of ECANS tuberculosis gene vaccine
[0148] Through the analysis of BLAST network database, DNAstar biological software and network database (http: / / www.syfpeithi.com / scripts / MHCServer.dll / home.htm), comprehensive hydrophilicity and hydrophobicity, softness, antigen index, surface accessibility, and HLA class I, II molecular binding, and other parameters, predicted four T cell epitopes obtained: the 189-228 gene of Mycobacterium tuberculosis ESAT-6 protein (EAST-6 189-228 ); the 369-405 gene of Mycobacterium tuberculosis Ag85A protein (Ag85A 369-405 ); the 162-207 gene of Mycobacterium tuberculosis CFP-10 protein (CFP10 162-207 ); the 420-459 gene of Mycobacterium tuberculosis Ag85B protein (Ag85B 420-459 ).
[0149] Using pcDNA3.1 or pVAX as the plasmid vector and the Mycobacterium tuberculosis HSP65 gene as the chimeric epitope gene carrier, on t...
Embodiment 2
[0150] The construction of embodiment 2pcDNA3.1-ECANS tuberculosis gene vaccine
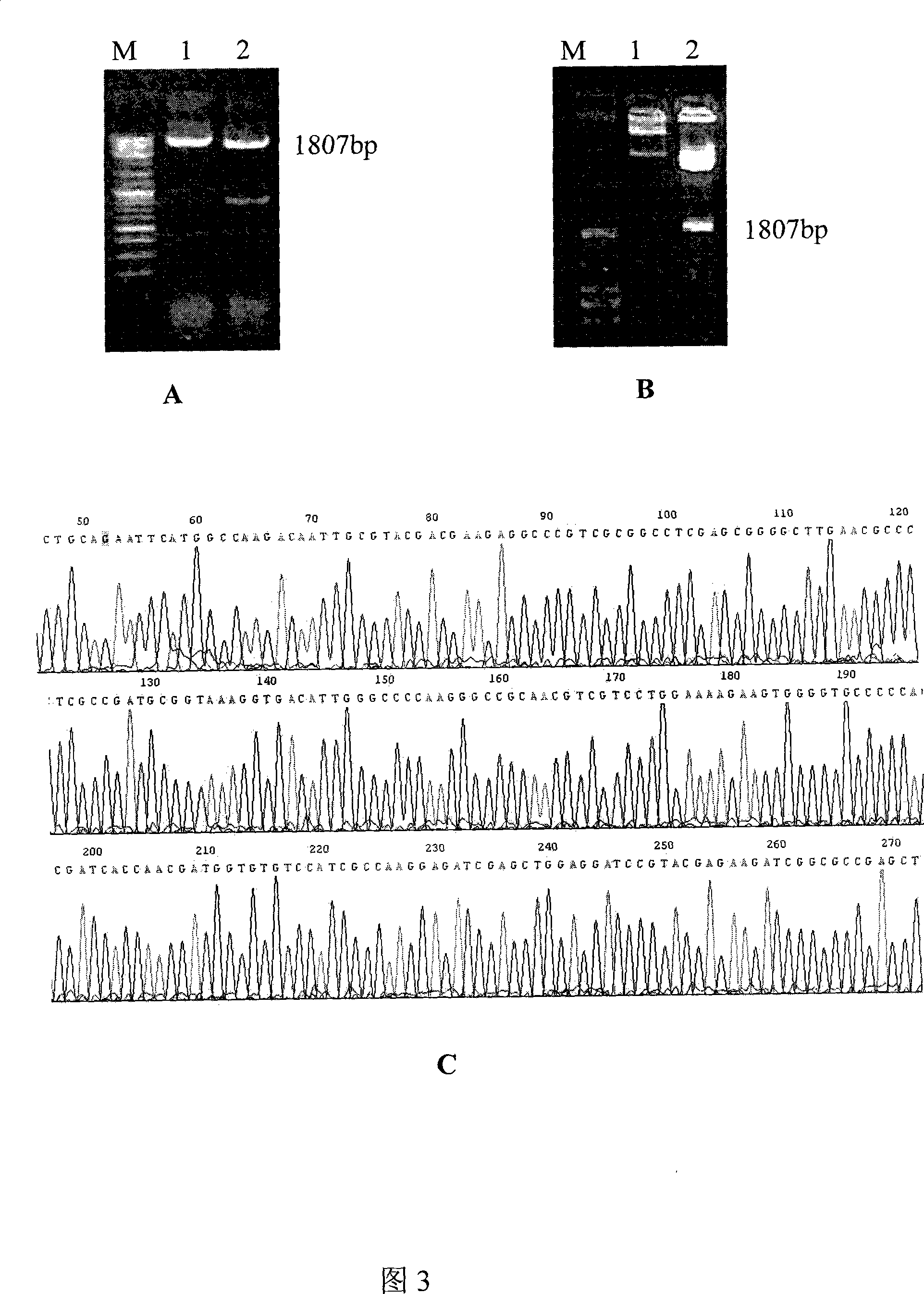
[0151] In order not to introduce a restriction site between the HSP65 gene and the T cell epitope gene, the present invention utilizes the method of direct synthesis of DNA primers and PCR to sequentially amplify three sections of partially overlapping HSP65 gene from the 5' and 3' ends The gene fragments of T cell epitopes and T cell epitopes were denatured and connected by overlapping complementary sequences, and finally the HSP65 full-length gene chimerized with 4 T cell epitopes was amplified by PCR with 5' and 3' HSP65 primers. At the same time, EcoR I and Hind III restriction sites are respectively placed at both ends of the gene, and can be connected into vector pcDNA3.1(-) or prokaryotic expression vector pET32a after double digestion.
[0152] First extract the DNA of Mycobacterium tuberculosis H37Rv strain (Shanghai Center for Disease Control and Prevention, Department of Tuberculosis) ...
Embodiment 3
[0221] Example 3 Construction of pET32a-ECANS prokaryotic expression vector and protein expression and purification
[0222] The amplified ECANS coding gene fragment or HSP65 gene was double-digested with EcoR I and Hind III, and connected with the corresponding prokaryotic expression vector pET32a to construct pET32a-ECANS and pET32a-HSP65 prokaryotic expression plasmids.
[0223] Escherichia coli BL21(DE3) competent cells were transformed with pET32a-ECANS, cultured overnight at 37°C, and positive clones were screened. Shake culture in LB (Amp100μg / ml) liquid medium until A600 reaches about 0.75, and add isopropylthiosemiglucoside (IPTG) to a final concentration of 0.5mM. Continue shaking and culturing for 3 hours, collect the bacteria by centrifugation at 4000r / min for 20min, resuspend in 1×PBS, sonicate, centrifuge at 12000r / min for 20min at 4°C, and harvest the supernatant and precipitate respectively. The supernatant was passed through an affinity chromatography column ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com