Cromoglyn sodium eye drops without bacteria inhibitor and preparation method thereof
A technology for sodium cromoglycate and eye drops, applied in the field of sodium cromoglycate eye drops and preparation thereof, can solve problems such as high risk, achieve the effects of saving costs, avoiding toxic and side effects, and ensuring sterility requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
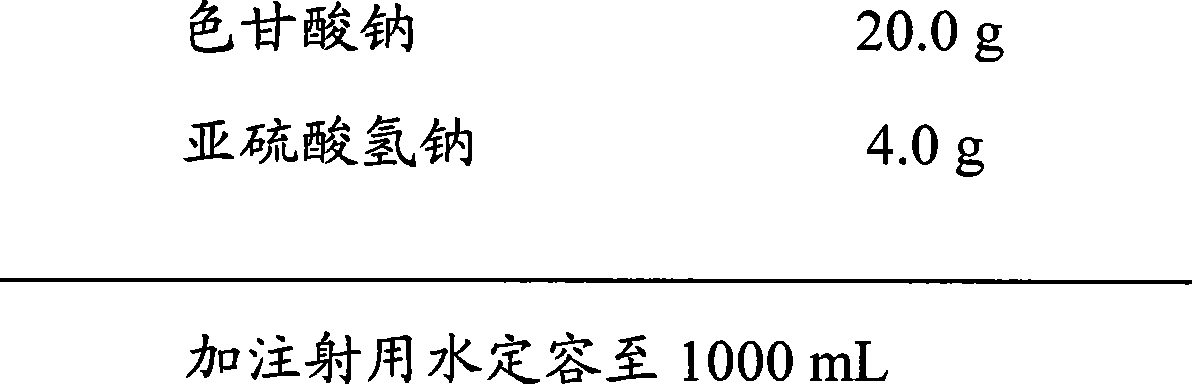
[0047] prescription:
[0048]
[0049] Its preparation method:
[0050] a. Precisely weigh 20.0 g of sodium cromoglycate and 4.0 g of sodium bisulfite, add 200 mL of water for injection to dissolve, stir evenly, and dilute to 1000 mL with water for injection to obtain a liquid medicine.
[0051] b. The above medicinal solution is filtered once through a 0.45 μm microporous membrane, and then filtered twice through a 0.22 μm microporous membrane under a Class 100 environment.
[0052] c. After the medicinal liquid is tested and passed, fill 0.4mL of the above medicinal liquid into a 1mL single-dose packaging plastic container in a class 100 environment, and seal it to obtain the finished product.
Embodiment 2
[0054] prescription:
[0055]
[0056] Its preparation method:
[0057]a. Accurately weigh 20.0g sodium cromoglycate, 7.24g sodium dihydrogen phosphate, 0.94g disodium hydrogen phosphate, 1.2g sodium bisulfite, add 200mL water for injection to dissolve, stir well, and dilute to 1000mL with water for injection, Get the medicine.
[0058] b. The above medicinal solution is filtered once through a 0.45 μm microporous membrane, and then filtered twice through a 0.22 μm microporous membrane under a Class 100 environment.
[0059] c. After the medicinal liquid is tested and passed, fill 2mL of the above medicinal liquid into a 3mL single-dose packaging plastic container under Class 100 environment, and seal it to obtain the finished product.
Embodiment 3
[0061] prescription:
[0062]
[0063] Its preparation method:
[0064] a. Accurately weigh 20.0 g of sodium cromolyn, 12.0 g of boric acid, 0.5 g of borax, and 1.0 g of sodium metabisulfite, add 200 mL of water for injection to dissolve, stir evenly, and dilute to 1000 mL with water for injection to obtain a liquid medicine.
[0065] b. Filter the medicinal solution prepared above through a 0.45 μm microporous membrane once, and then through a 0.22 μm microporous membrane once.
[0066] c. The filtered medicinal solution is sterilized at 121° C. for 8 minutes by autoclaving, and then cooled.
[0067] d. After the medicinal liquid is tested and passed, fill 0.5mL of the above medicinal liquid into a 1mL single-dose packaging plastic container in a class 100 environment, and seal it to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com