Hafnate luminescent material and preparation method thereof
A luminescent material, hafnium salt technology, applied in luminescent materials, chemical instruments and methods, sustainable manufacturing/processing, etc., can solve the problems of high energy consumption, long production cycle, and no obvious improvement in luminous performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Y(NO 3 ) 3 6H 2 O 4.7880g, HfOCl 2 11.6110g, 0.095mol / l Eu(NO 3 ) 3 Solution 4.0ml, C 2 h 5 NO 2 0.7820g, CH 4 N 2 O 0.9384g.
[0018] Weigh 4.7880g Y(NO 3 ) 3 6H 2 O powder and 11.6110 g HfOCl 2 After the powder was put into a beaker and dissolved in distilled water, 4.0ml of Eu(NO 3 ) 3 solution, measure the pH value of the solution, and adjust it to approximately equal to 1 with nitric acid. Put the beaker on the magnetic stirrer, add 0.7820g of glycine C to it while stirring 2 h 5 NO 2 and 0.9384g urea CH 4 N 2 O, and add 0.015 g of EDTA as a chelating agent, and stir vigorously for a while until the raw material is mixed with the fuel. Put the mixture in an oven at 200°C to evaporate the water, then put it into a muffle furnace and heat it to 450°C to make it foam and burn, and then heat it in a high-temperature furnace at 800°C for 2 hours to finally get the desired Luminescent material.
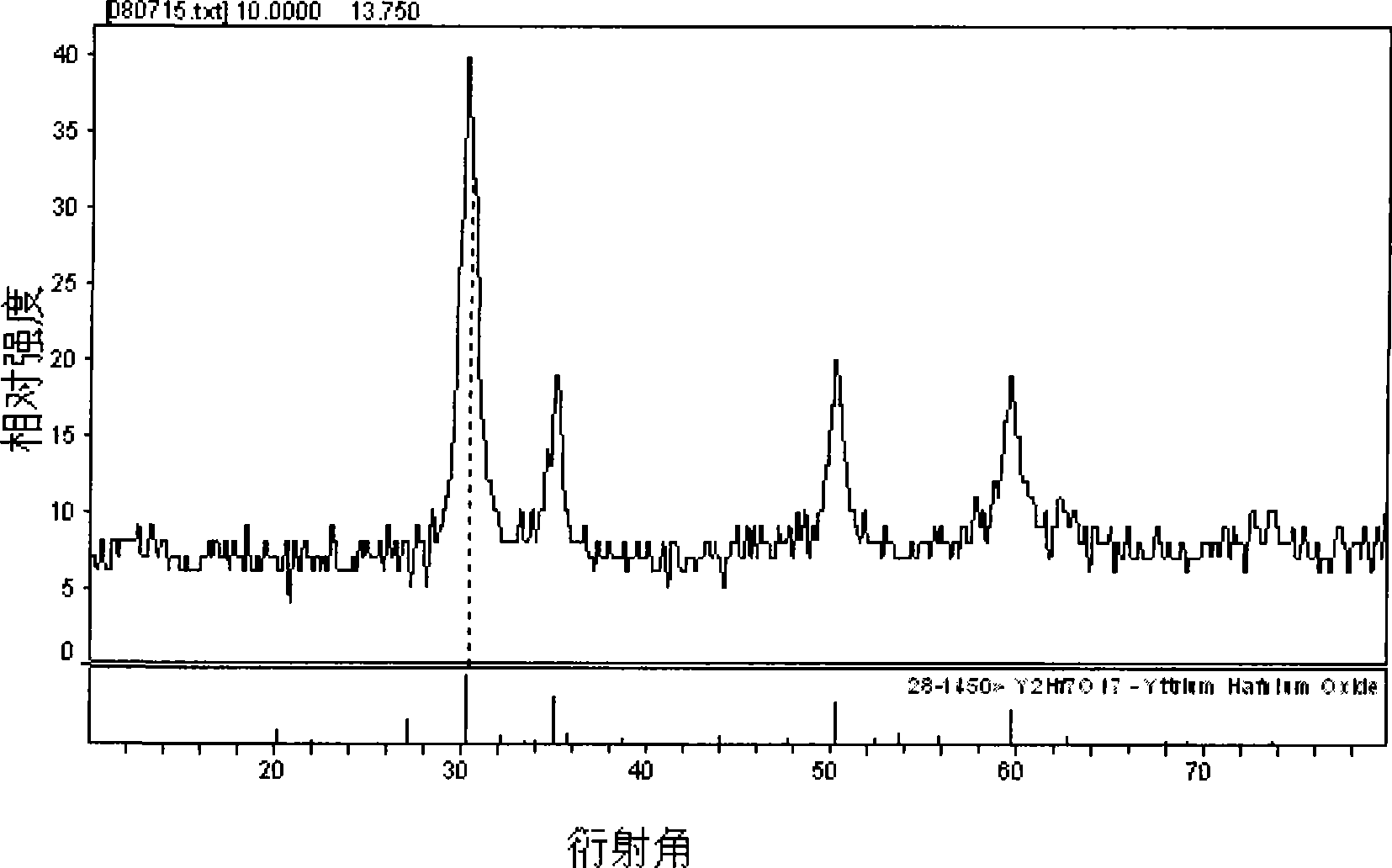
[0019] Referring to the accompanying drawings, the s...
Embodiment 2
[0021] Y(NO 3 ) 3 6H 2 O 4.7880g, HfOCl 2 11.6110g, 0.095mol / l Eu(NO 3 ) 3 Solution 6.0ml, C 2 h 5 NO 2 0.7820g, CH 4 N 2 O 0.9384g.
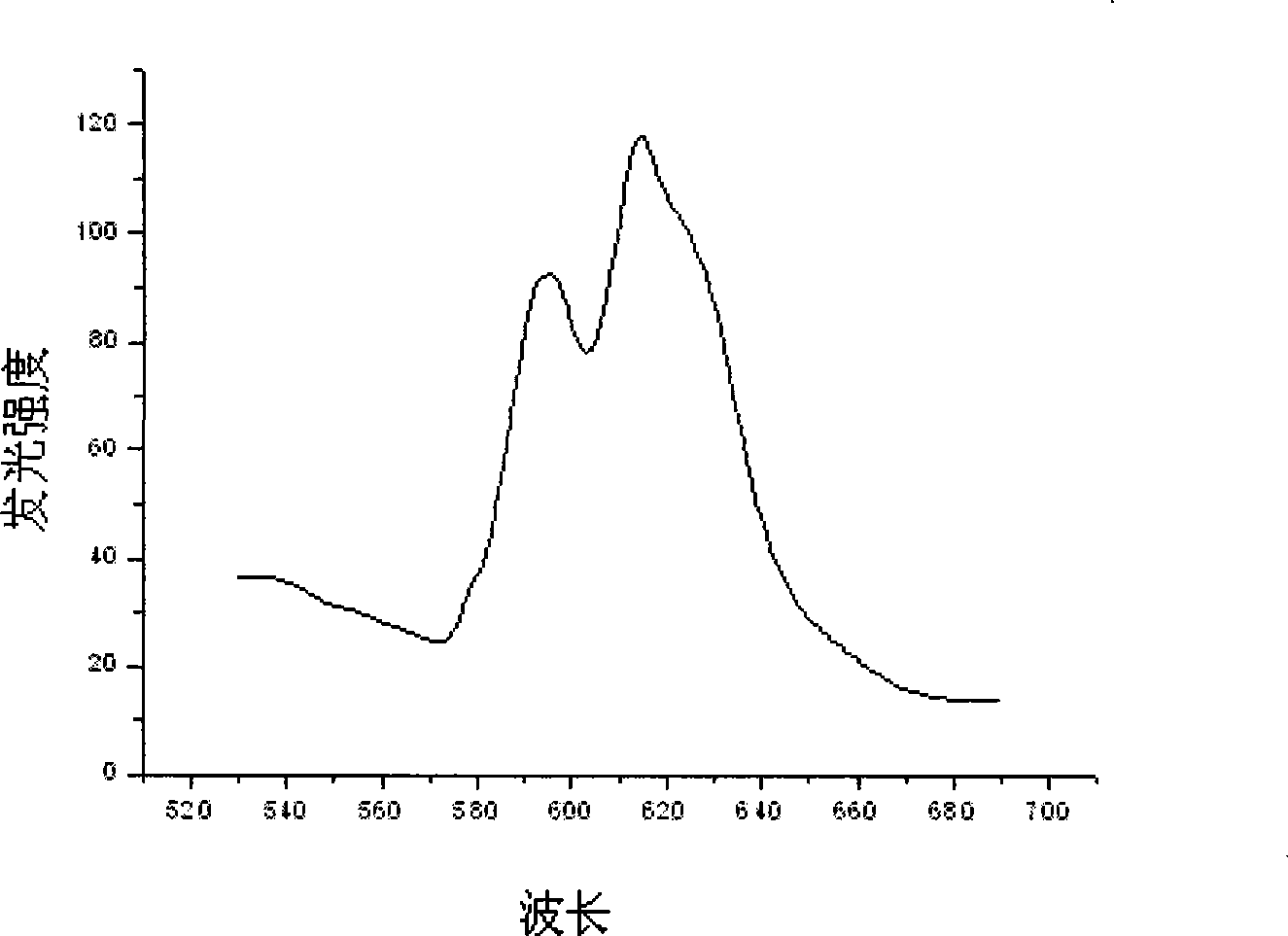
[0022] Weigh 4.7880g Y(NO 3 ) 3 6H 2 O powder and 11.6110 g HfOCl 2 After the powder was put into a beaker and dissolved in distilled water, 6.0ml of Eu(NO 3 ) 3 solution. Measure the pH of the solution and adjust it to approximately 1 with nitric acid. Put the beaker on the magnetic stirrer, add 0.7820g of glycine C to it while stirring 2 h 5 NO 2 and 0.9384g urea CH 4 N 2 O, and add 0.015 g of EDTA as a chelating agent, and stir vigorously for a while until the raw material is mixed with the fuel. Put the mixture in an oven at 200°C to evaporate the water, then put it into a muffle furnace and heat it to 450°C to make it foam and burn, and then heat it in a high-temperature furnace at 800°C for 2 hours to finally get the desired Luminescent material. The structure of the luminescent material synthesized in this exampl...
Embodiment 3
[0024] Y(NO 3 ) 3 6H 2 O4.7880g, HfOCl 2 11.6110g, 0.095mol / l Eu(NO 3 ) 3 Solution 8.0ml, C 2 h 5 NO 2 0.7820g, CH 4 N 2 O0.9384g.
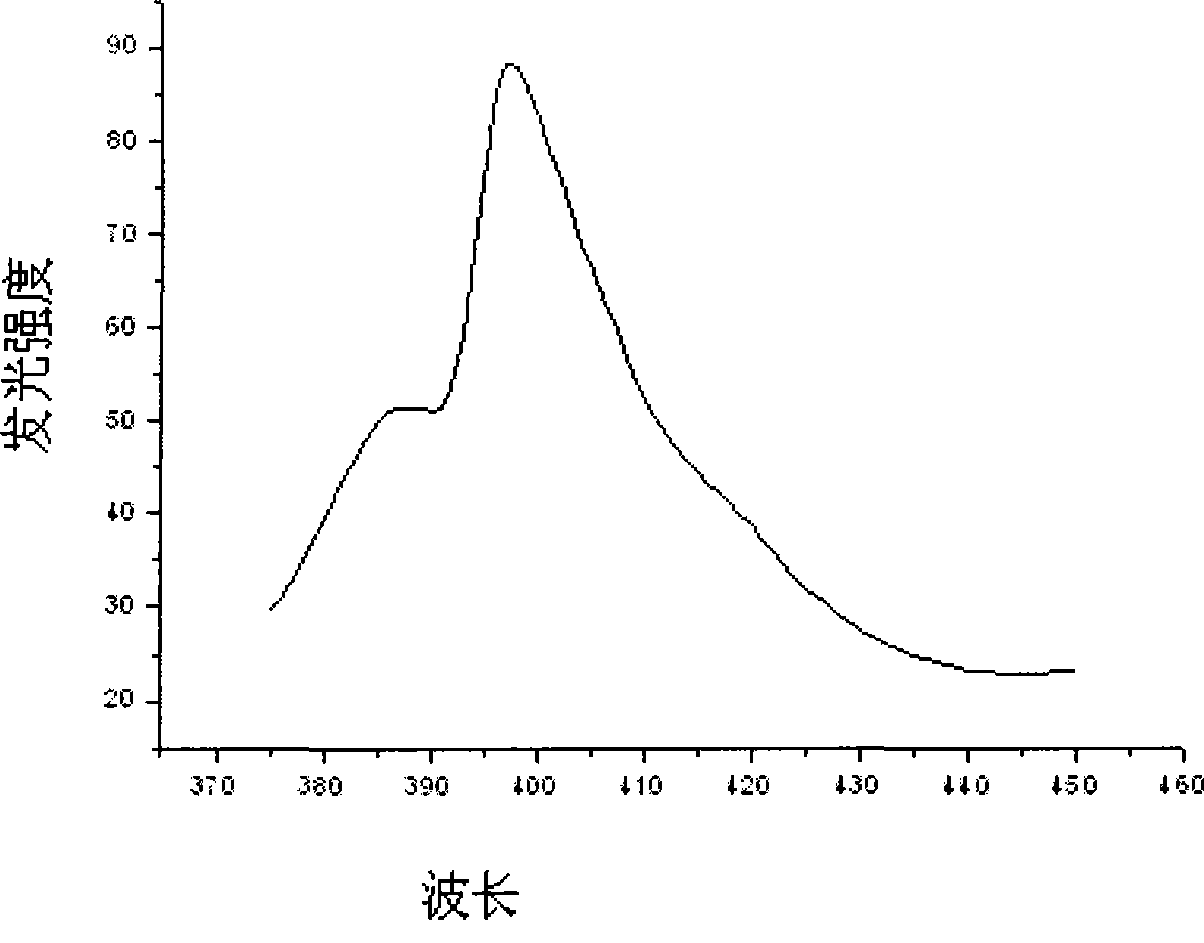
[0025] Weigh 4.7880g Y(NO 3 ) 3 6H 2 O powder and 11.6110 g HfOCl 2 After the powder was put into a beaker and dissolved in distilled water, 8.0ml of Eu(NO 3 ) 3 solution. Measure the pH of the solution and adjust it to approximately 1 with nitric acid. Put the beaker on the magnetic stirrer, add 0.7820g of glycine C to it while stirring 2 h 5 NO 2 and 0.9384g urea CH 4 N 2 O, and add 0.015 g of EDTA as a chelating agent, and stir vigorously for a while until the raw material is mixed with the fuel. Put the mixture in an oven at 200°C to evaporate the water, then put it into a muffle furnace and heat it to 450°C to make it foam and burn, and then heat it in a high-temperature furnace at 800°C for 2 hours to finally get the desired Luminescent material. The structure of the scintillation material synthesized in this example...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com